Enantiomers – One body with two souls!
by egpat 25 Mar 2019
Have you ever guessed that such compounds called enantiomers exists in nature which are identical in melting point, boiling point, solubility and even act quiet similarly in their reactions both in rate and extent but still exist as two different compounds? You know twins and even you can find your right and left hands both looking similar still you can find some point of difference in their functionality. For instance whatever the activities you do with your right hand are quite different with that of right hand. But the story of enantiomers is something different!
It’s astonishing that in the organic chemistry a pair of compounds , termed as enantiomers, exist being completely identical in all aspects except in one property, the later being their optical property.
Quiet interesting!
So these are the compounds with one body with two souls!
What makes them to be so much identical?
Simply the neighbours around the carbon that arranged differently to bring the unique property to them – optical activity!
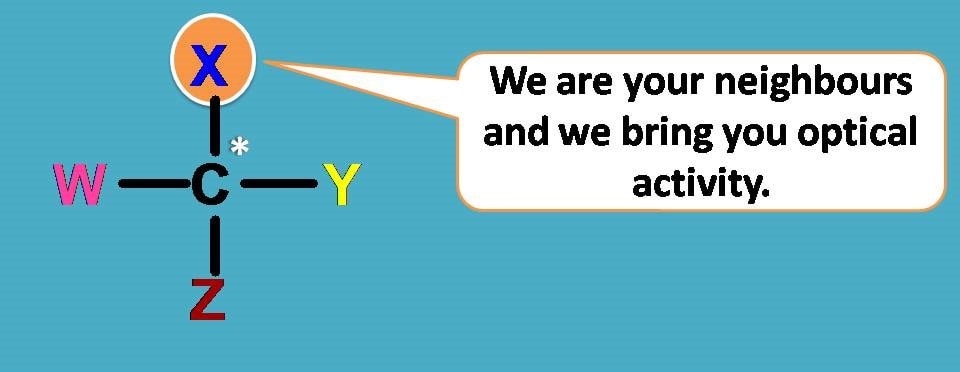
It’s great!
Can anyone be there with that carbon to celebrate this moment?
Why not? Anyone can participate, but there is a restriction. One can join their hand with carbon only one time.
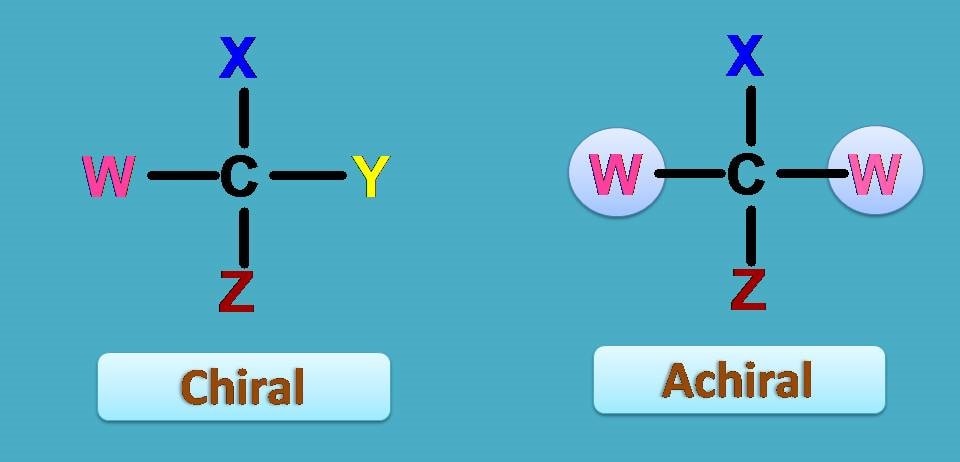
In another words that carbon which bears four different groups is chiral responsible for optical isomerism and existence of enantiomers.
Enantiomers are mirror images
By this time we know that enantiomers are the pair of compounds look and act alike with different optical properties.
But another interesting feature is that they are mirror images of each other. Even we can’t find any difference in their physical properties still their orientation in the space is different, just like your left and right hand, and exist as mirror images.
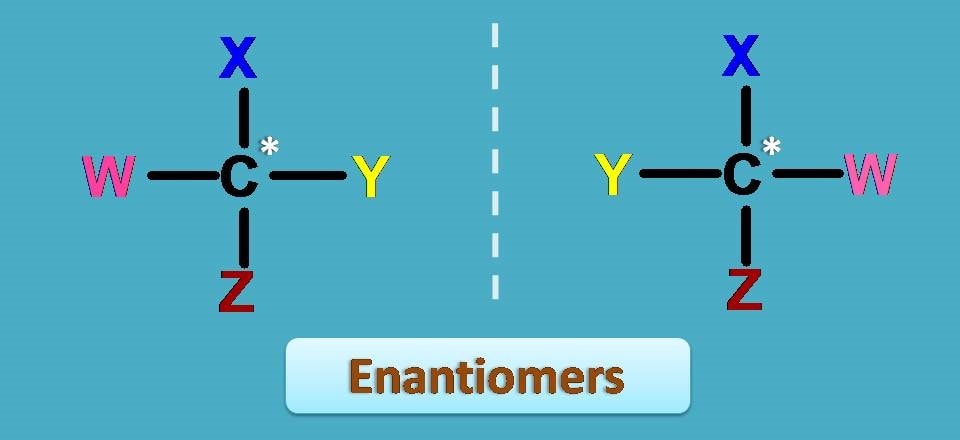
Suppose the central vertical dotted line is the mirror, you can find the groups are at equal distance from the mirror in both the structures.
Enantiomers are non-super imposable
Now a small doubt may peep into your mind that, all mirror images are different? Certainly, not all. Let’s have a square here.
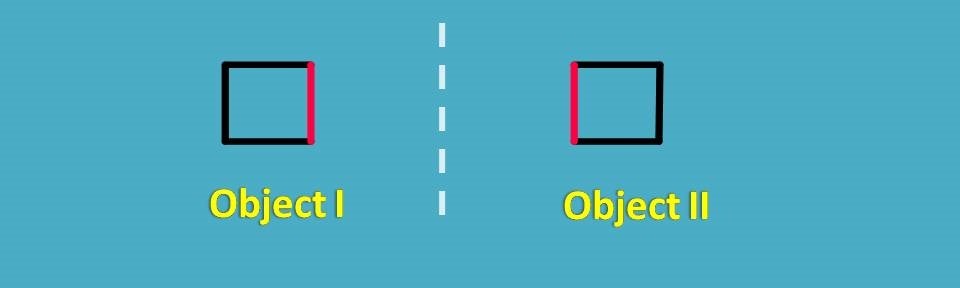
It can have its mirror image and you can easily convert object 1 into object 2 simply rotating by 180o.
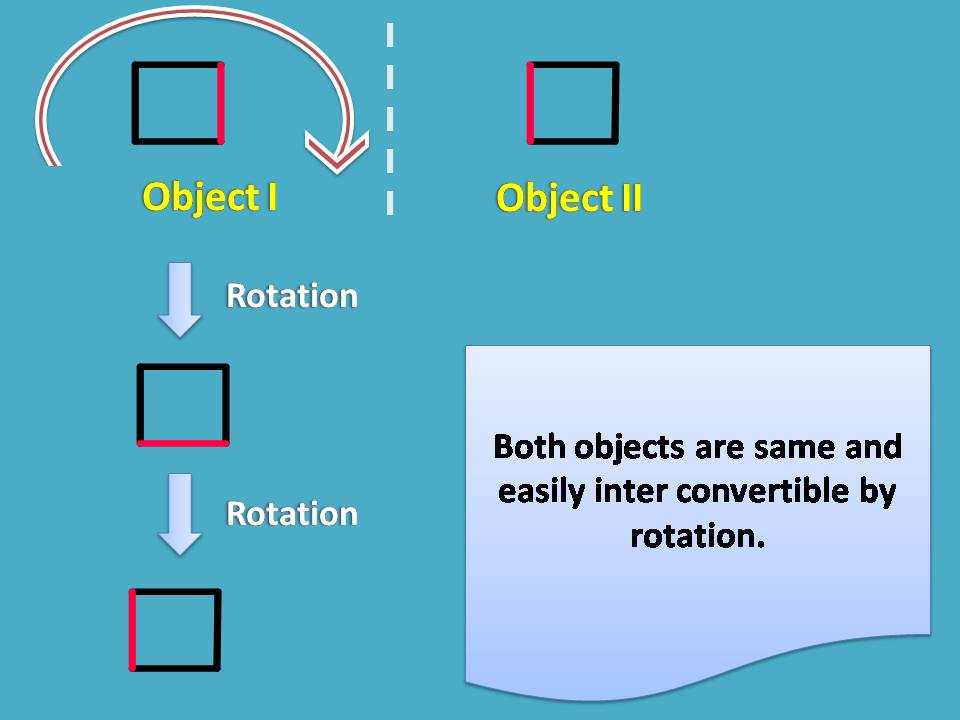
So they are not different objects at all. Now let’s take another square with few attachments.
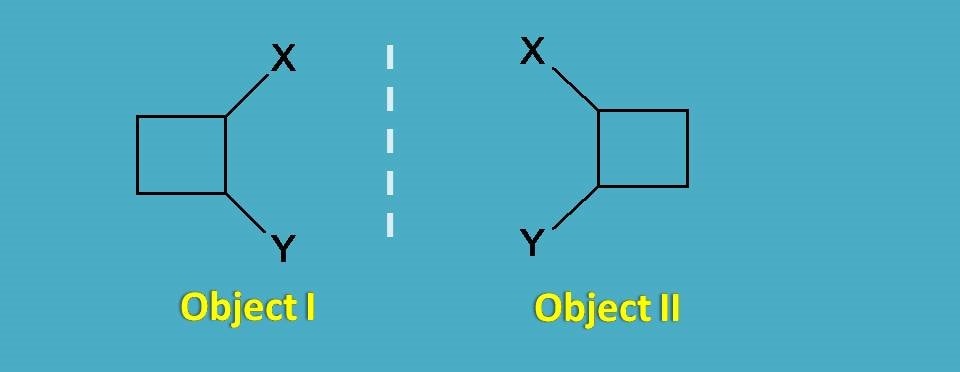
Again you can find here mirror images and let’s check for any superimposability.
In this case, rotation of object I by 180o will not produce object II as the square has few attachements which differ in positions.
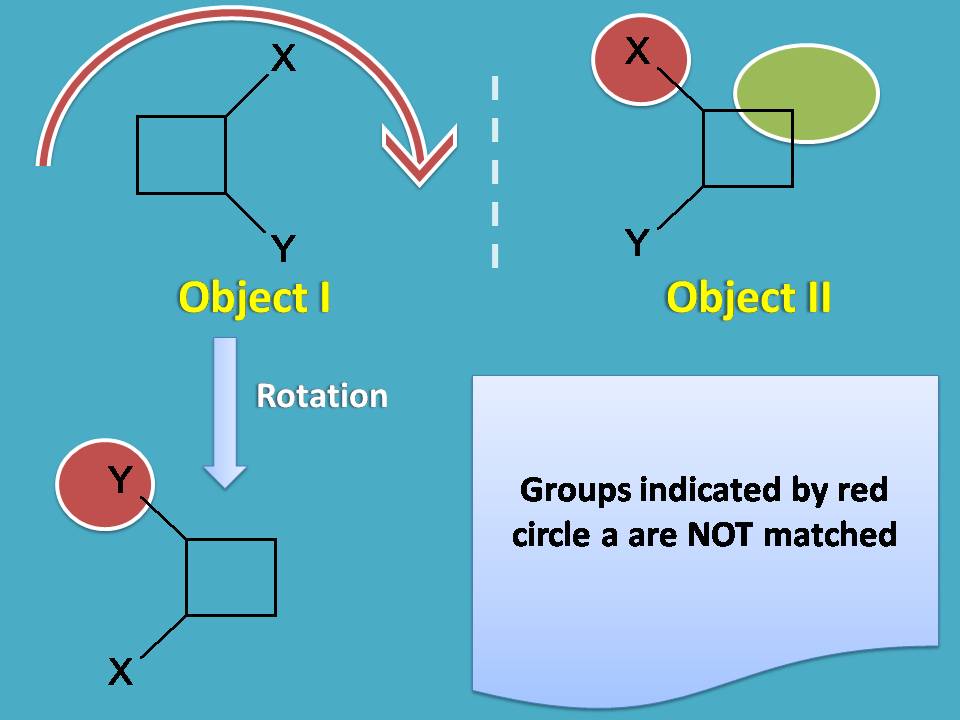
Now let's check the superimposability by flip of the object. Let's flip the object I horizontally. Ahh! We got the same object, so both are not different but same.
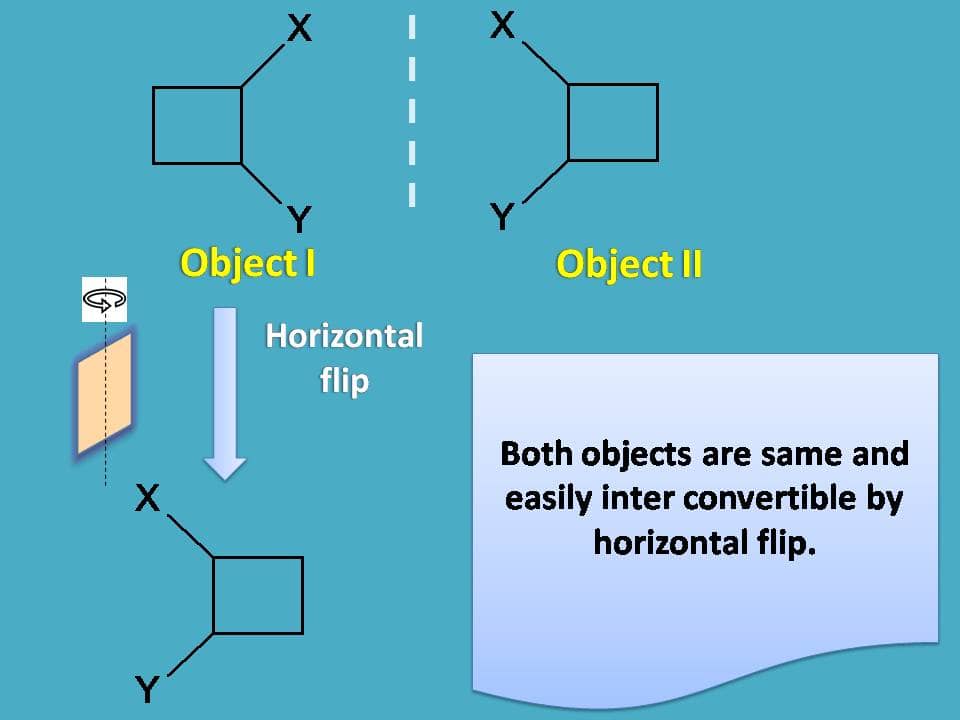
That’s nice. We proved that both objects are not different but of course this time with more efforts doing both rotation and flip.
Now let’s turn to the enantiomers and see the power of chiral carbon.
Chiral carbon in the enantiomers is not like that square we have seen above that exists within the plane. The hybridisation of carbon in the enantiomers is sp3 and it has tetrahedral shape.
To understand the concept well, let’s use perspective formula here. Suppose we indicate the groups within the plane with solid lines and the group above the plane with solid wedge and that group below the plane with hatched wedge.
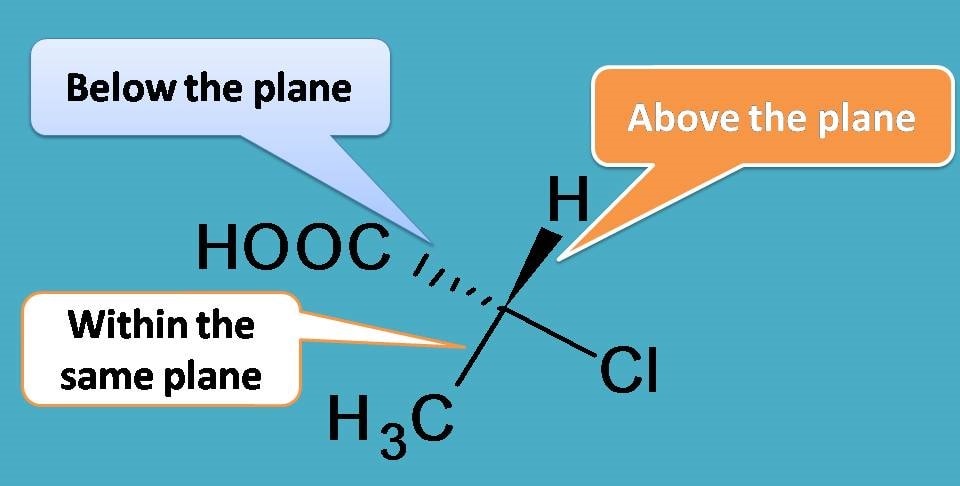
Due to tetrahedral nature, if we orient the molecule such that two groups are within the plane, then other two groups will be out of the plane, one being above and other below the plane.
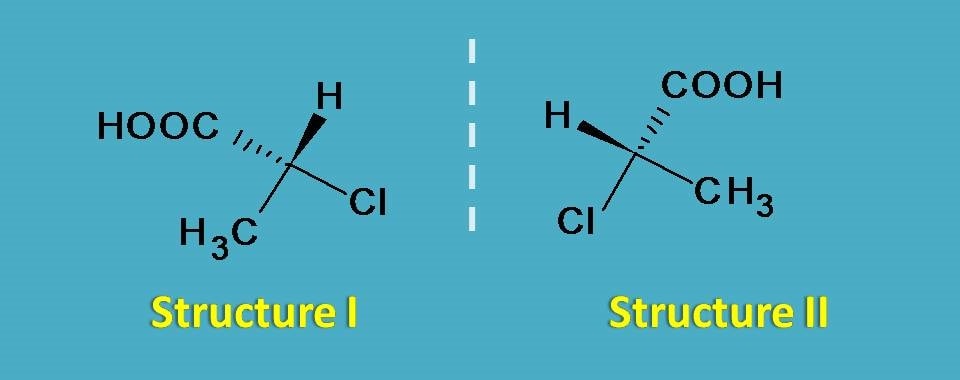
Now let’s check for superimposability.
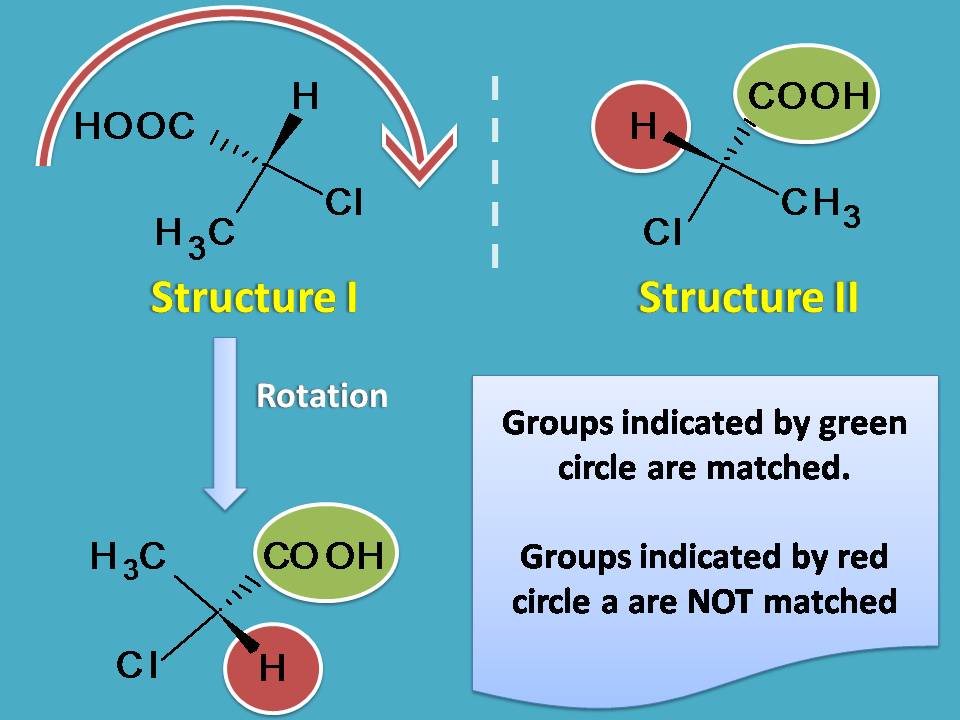
Suppose if you rotate structure I by 180o you will get a new orientation which is not similar to structure II. You can find difference at orientation of hydrogen in the two structures. So these structures are not inter-convertible by rotation.
Now let's check their superimposability by flip. Again by flipping of structure I horizontally you will get another orientation which may appear similar to structure II but in reality it is different. You can clearly observe that in the flipped structure carboxylic acid group is attached above the plane while in structure II it is below the plane. You can also find similar difference at hydrogen. So even by horizontal flip still you will not get the same structure.
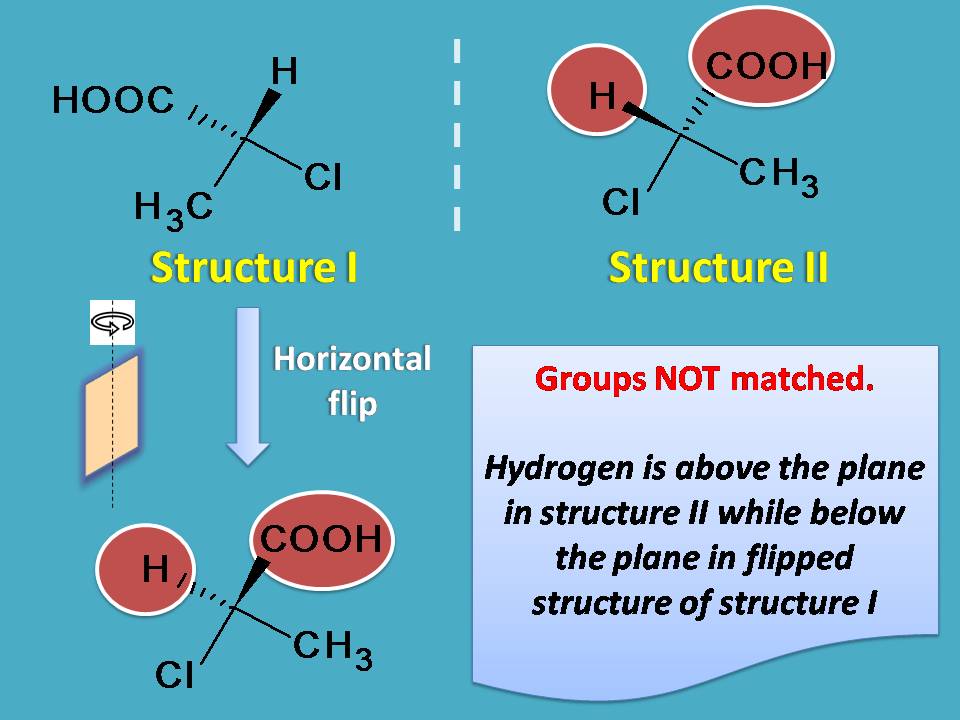
In another words, they are not same compounds and therefore exist as enantiomers.
Conclusion
Enantiomers are pair of optical isomers which are completely identical in their physical and chemical properties still exist as separate compounds. So it is not easy to separate these compounds. In view of their structure, they have same connectivity but differ in arrangement resulting in non-superimposable mirror images. For a compound to exhibit optical activity and exist as pair of enantiomers it should have a chiral carbon attached with four different groups.