- Home >
- Test papers >
MCQ on ESR spectroscopy: Page-1
(A) Free radicals
(B) Paramagnetic materials
(C) Transition metals
(D) Diamagnetic materials
Electron spin resonance is shown by compounds having unpaired electron. Electron has spin angular momentum as ½ and in case of paired electrons, the net spin will be zero as the electrons are paired with opposite spin. So ESR signal can’t be observed in diamagnetic materials where all the electrons are paired with opposite spin.

(A) R and S
(B) P and Q
(C) Q and R
(D) P and S
Both Tesla and Gauss are the units for magnetic field strength. Tesla is the SI unit whereas Gauss is CGS unit.
(A) IR radiation
(B) Radio waves
(C) X-radiation
(D) Microwaves
ESR spectra of a free radical were observed as shown below. The hyperfine splitting observed in the spectra may be due to a single nuclei of
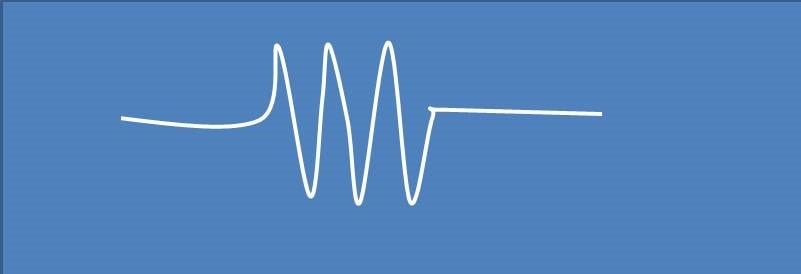
(A) 1H
(B) 12C
(C) 19F
(D) 14N
Spectra shown above contains 3 peak splitting of electron resonance line. It is possible by either with a nuclei I=1 or with three equivalent nuclei with I=½ . Among the options given, nitrogen has I=1, so it brings 3 splitting of resonance line.
For an unbound electron, value of Lande’ factor (g-factor) will be equal to
(A) 1.0098
(B) 2.0023
(C) 3.0015
(D) 6.0621