Directly acting cholinergic agents
As discussed earlier, these agents act as agonists directly on acetylcholine receptors.
But on which receptor they act? muscarinic or nicotinic?
Except carbachol, other two drugs such as bethanechol and pilocarpine are more selective for muscarinic receptor and can be considered as muscarinic agonists. This selectivity towards muscarinic receptor can be produced by few structural changes to the acetylcholine.
So, let's compare structures of these drugs with acetylcholine.
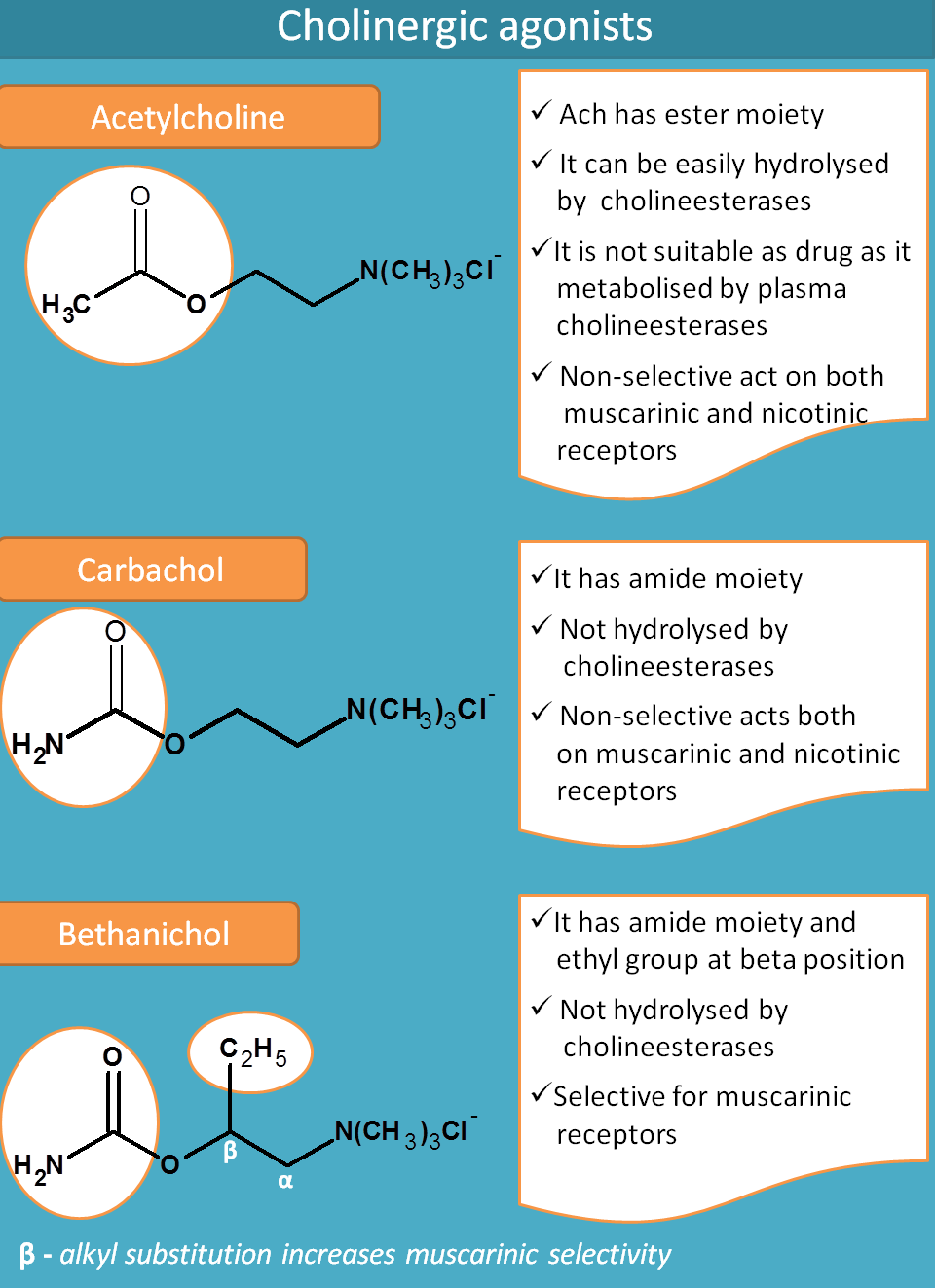
Acetylcholine
Acetylcholine is an endogenous mediator that contains two structural moieties.
- Acetyl group
- Choline moiety
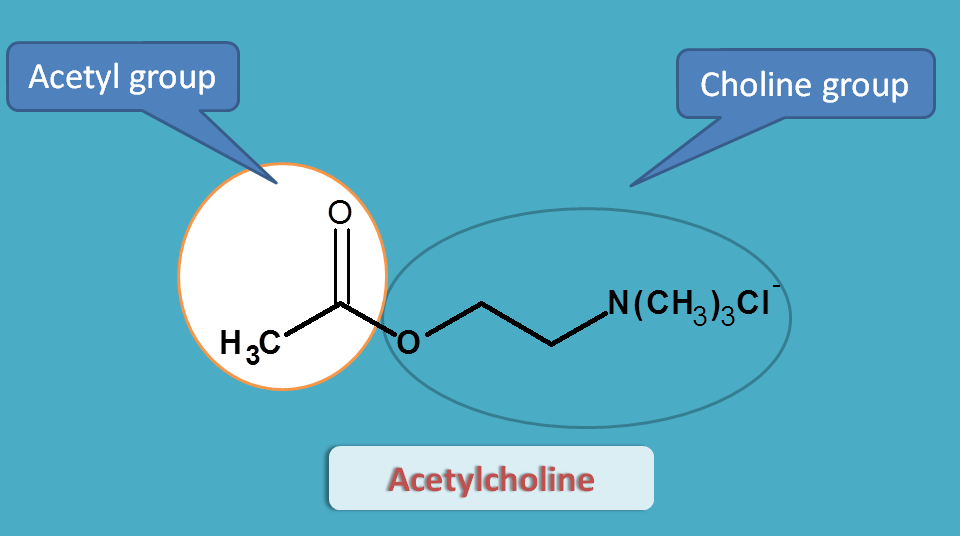
Acetylcholine can't be used as drug for two reasons.
- It is metabolised by plasma cholinesterases
- It is rapidly hydrolysed by acetylcholinesterase at cholinergic neurons
Acetylcholine after release into synaptic cleft binds to the cholinergic receptors. But it is rapdily cleaved as choline and acetate by acetylcholinesterase and made inactive at cholinergic nerve terminals.
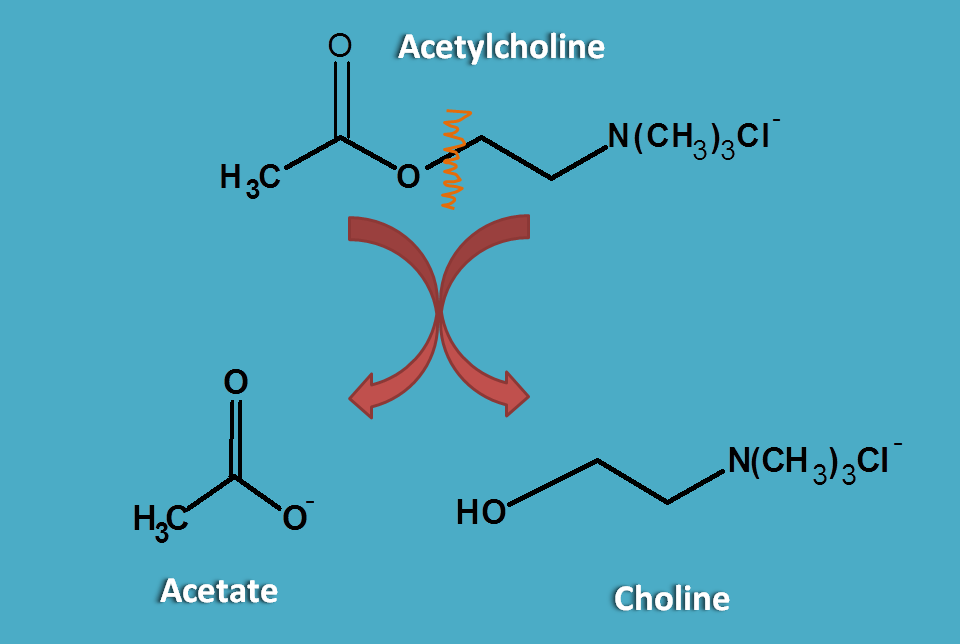
So a drug to act as cholinergic agonist it should be resistant to cholinesterases and not easily metabolised. This can be observed in carbachol.
Carbachol
Since the acetylcholine is cleaved at acetyl group, the later is replaced with an amide group in carbachol. Now with amide group (also called as carbamide group), carbachol is resistant to hydrolysis by acetlycholinesterases.
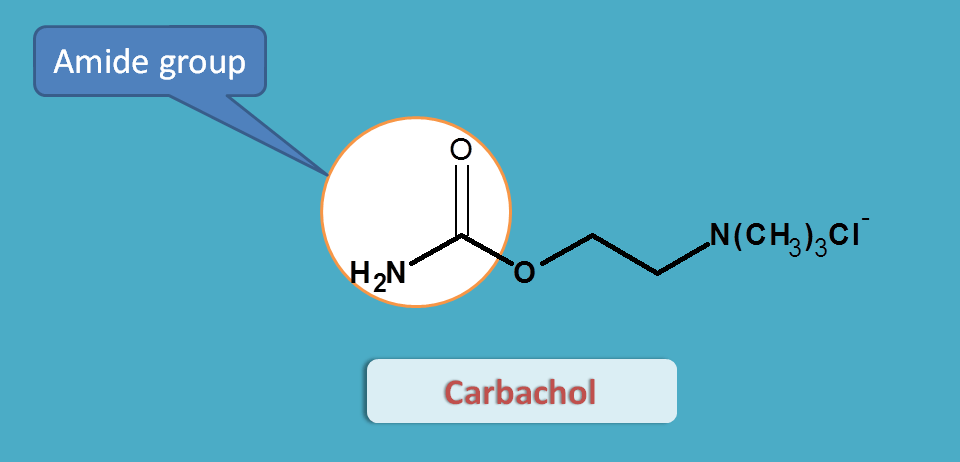
But still it is non-selective in action. Just like acetylcholine, it acts both on muscarinic and nicotinic receptors.
Bethanechol
Bethanechol is both resistant to hydrolysis by acetylcholinesterase and even selective for muscarinic receptors.
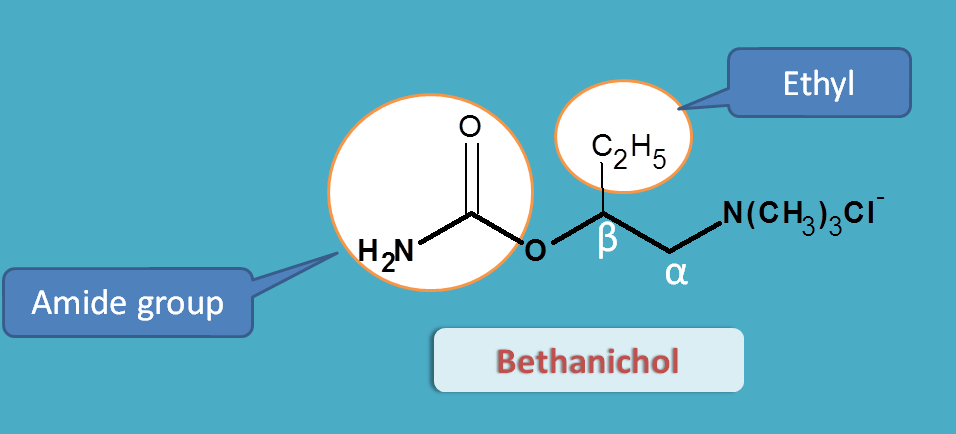
Why carbachol is non-selective but bethanechol is selective?
Again this is due to structural change. If you see the structure, bethanechol has an extra ethyl group at beta position. That’s why the name Beta + ethyl + choline=Bethanechol
According to Structural activity relationship (SAR) of cholinergic agonists,
α-alkyl substitution increase nicotinic selectivity whereas β-alkyl substitution increases muscarinic selectivity
Since betahichol has beta ethyl substitution it is more selective for muscarinic receptors
We can summarise these three agents as below.
Pilocarpine
Pilocarpine is a natural alkaloid obtained from Pilocarpus jaborandi.It's structure has no relation with the structure of acetylcholine but it was naturally found to act as muscarinic agonist.
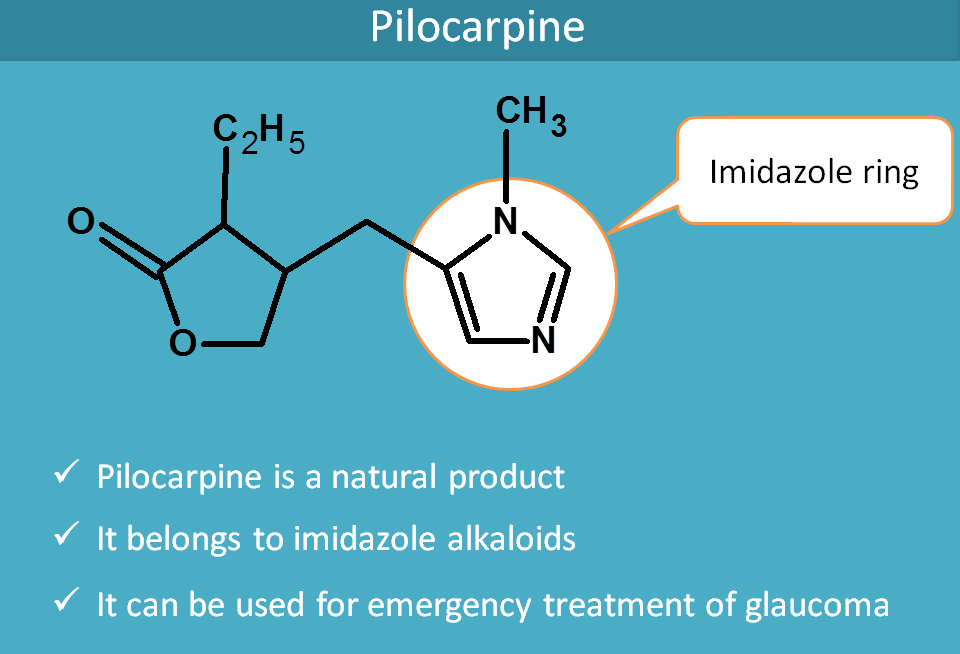
It belongs to imidazole alkaloids and you can easily observe the imidazole ring in its structure.
Here is a video on drug targets acting on muscarinic receptors.
How they act?
These drugs have more affinity towards cholinergic receptors and once bound they are not easily removed by acetylcholinesterase. Therefore they bind for long period compared with acetylcholine and produce pharmacological response similar to that produced by acetylcholine.