How to write IUPAC Name of sulfonamides
by egpat 08-07-2017
Sulfonamides are one of the oldest antibacterial agents that were developed for various types of bacterial infections. Since the entry of antibiotics into the medical world, sulfonamides usage was somewhat declined but still these drugs are useful in ophthalmic infections, urinary tract infections and even for treatment of burns and wounds.
In this article, we will discuss how to write IUPAC name of sulfonamides.
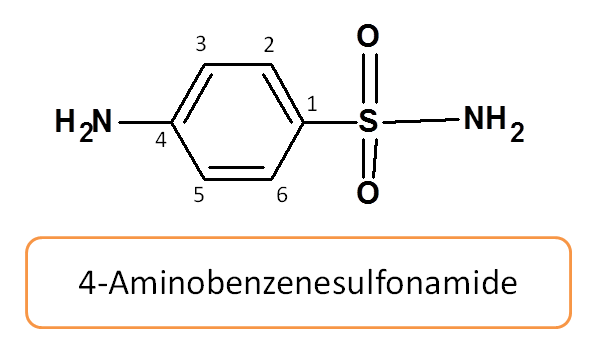
Let’s start with simple sulfonamide i.e. sulfanilamide
Working Example 1: Sulfanilamide
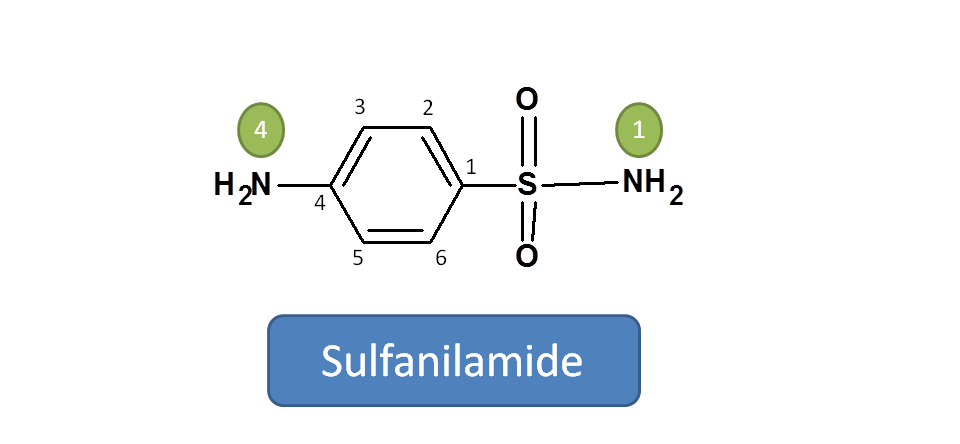
Step 1: Identify the principal functional group
Here two functional groups are present. One is sulfonamide and another is amine group. 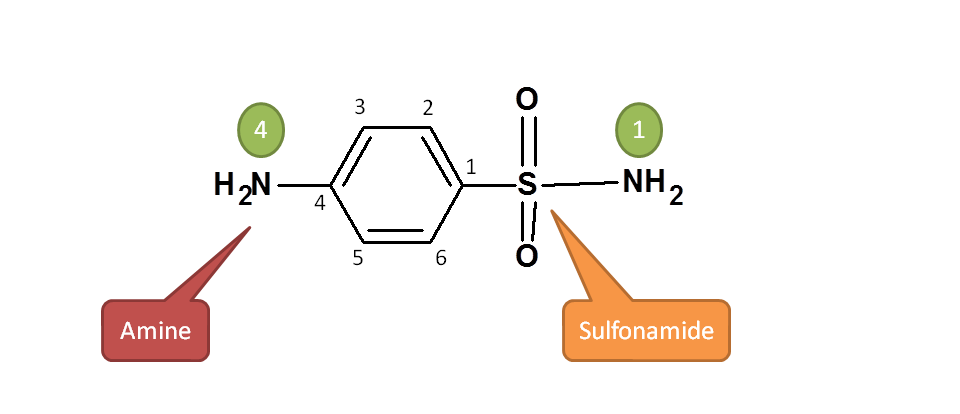 According to the priority order of functional groups with respect to IUPAC naming, sulfonamide should be given more preference over amine group. Hence the principle functional group is sulfonamide attached to aromatic ring.
According to the priority order of functional groups with respect to IUPAC naming, sulfonamide should be given more preference over amine group. Hence the principle functional group is sulfonamide attached to aromatic ring.
Step 2: Identify the parent chain and give the root name
Since the principal functional group is present on benzene, the later acts a parent chain. Hence the root name is benzene sulfonamide.
Step 3: Give numbering to the structure
Numbering should be done according to the following rule.
Numbering should be done from that direction which gives least possible number to the principal functional group.
Here benzene sulfonamide is the parent chain and numbering should be started from sulfonamide.
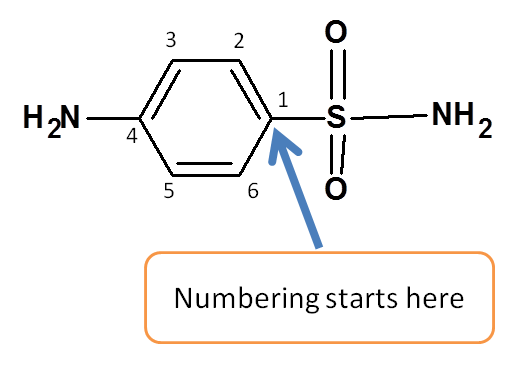
Step 4: Identify the side chains and indicate their location
Now let us see side chains. Sulfanilamide has an amine side chain at para position of the benzene ring.
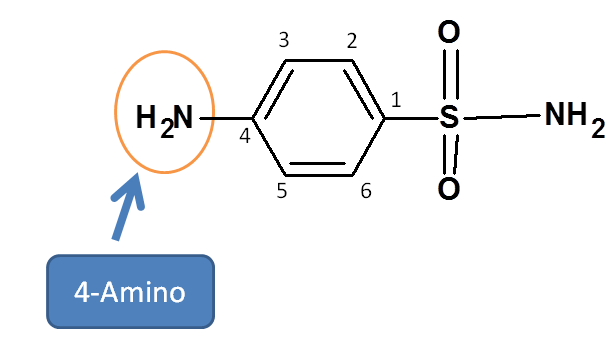
Hence prefix is 4-Amino
Step 5: Now arrange the side chains in alphabetical order.
Only one side chain is present so no need of arranging in alphabetical order.
Step 6 : Complete the name
Hence the IUPAC name of sulfanilamide is
Working Example 2: Sulfamethoxazole
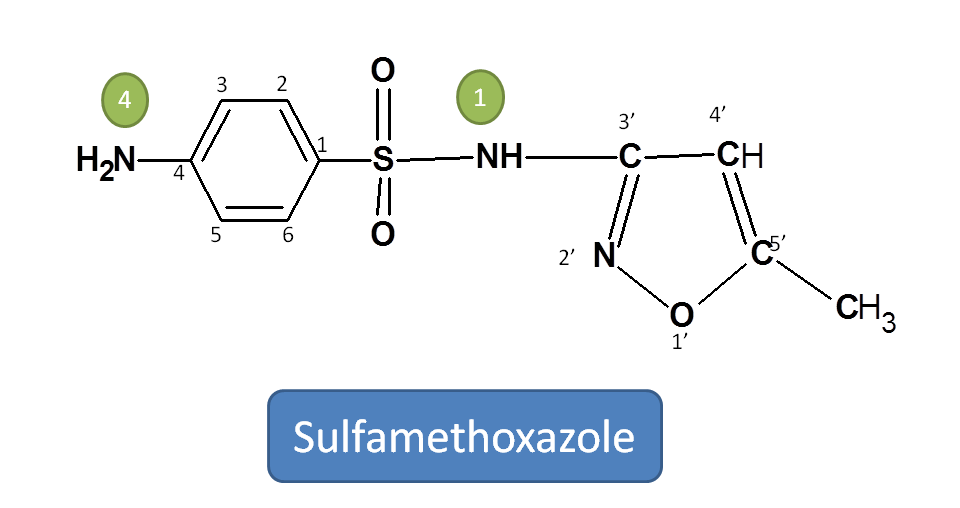
Step 1: Identify the principal functional group
Here again two functional groups are present viz. sulfonamide and amine.' As similar with the previous example, the principle functional group is sulfonamide attached to benzene.
Step 2: Identify the parent chain and give the root name
Now we can observe two ring systems. One is benzene and another is 1,2-oxazole.
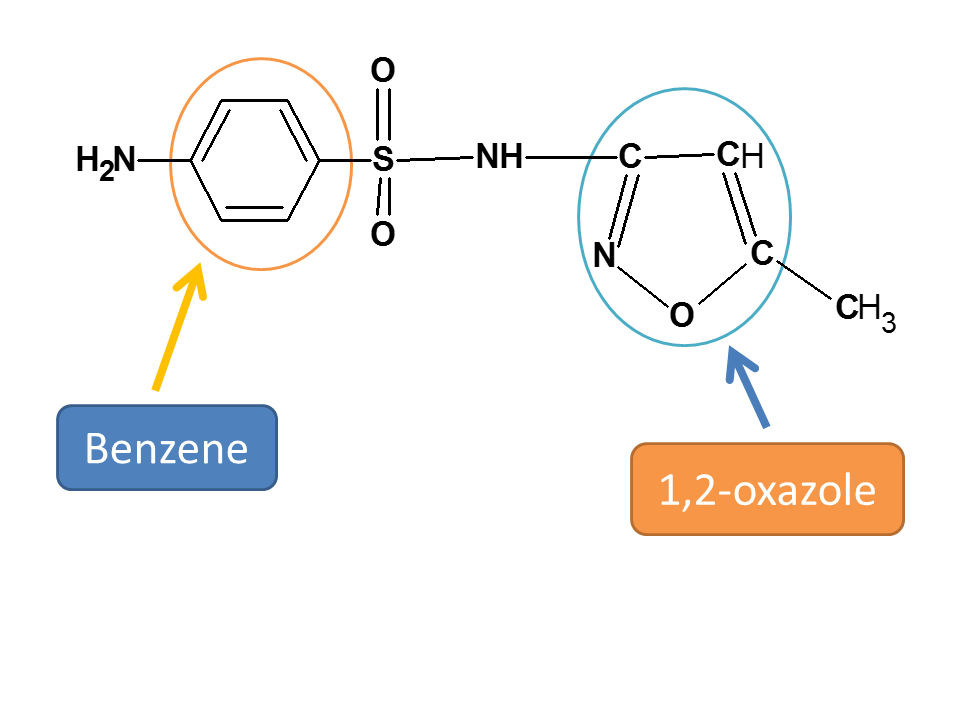
Since sulfonamide is present on benzene, it should be selected as parent chain.
Hence root name of the compound is benzenesulfonamide.
Step 3: Give numbering to the structure
Again numbering should be started from sulfonamide group on benzene.
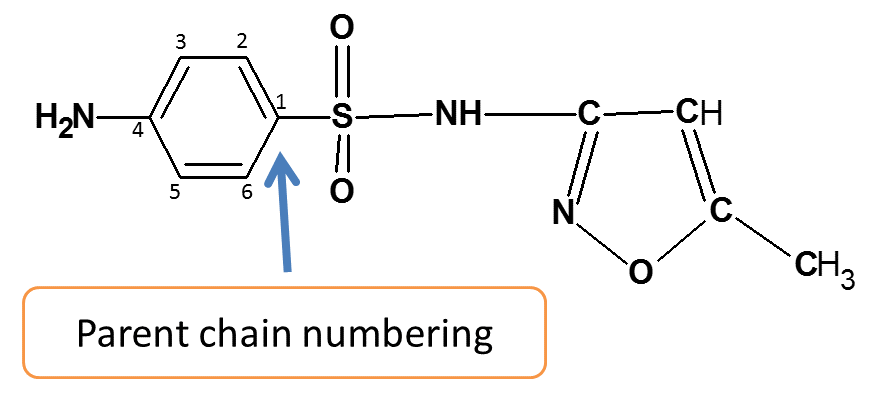
Step 4: Identify the side chains and indicate their location
Now let’s identify side chains. One side chain is amino group at para position of benzene and another is a heterocyclic ring on amine of the sulfonamide group.
One of the side chain is therefore 4-Amino.
Second side chain is the heterocyclic ring 1,2-oxazole and its numbering starts from oxygen. 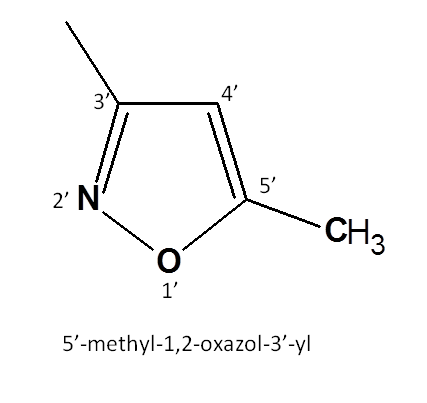
It has methyl side chain at 5th position therefore ring is
5'-methyl-1',2'-oxazole
It is attached to main chain by 3rd position. Hence the suffix “e” should be replaced by “yl” with its location. Therefore, name of the side chain is now
5'-methyl-1',2'-oxazol-3'-yl
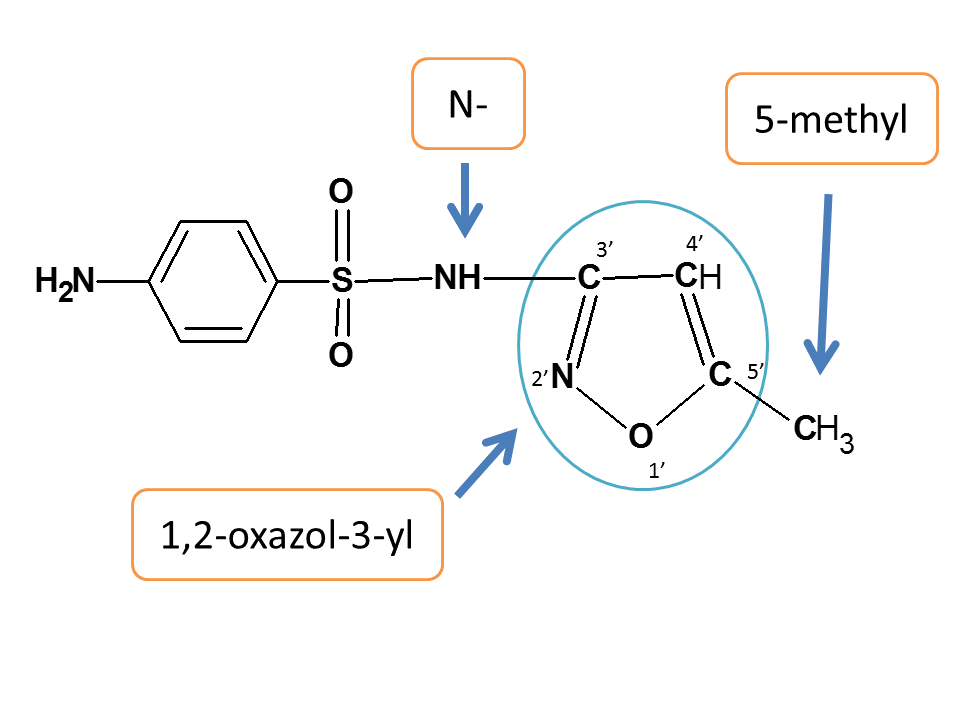
This entire chain is attached to amine group of benzenesulfonamide.
N-[5'-methyl-1',2'-oxazol-3'-yl]
Step 5: Now arrange the side chains in alphabetical order.
Let's arrange all the prefixes in alphabetical order.
4-Amino
N-[5'-methyl-1',2'-oxazol-3'-yl]
Step 6 : Complete the name
Hence the IUPAC name of sulfamethoxazole is
4-Amino-N-[5'-methyl-1',2'-oxazol-3'-yl] benzesulfonamide
Working Example 3: Sulfadiazine
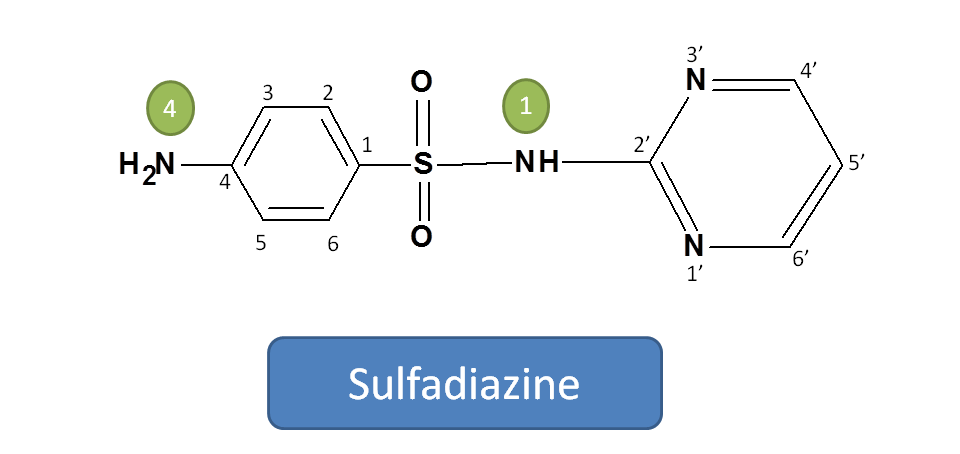
Step: 1,2 and 3
Step 1,2 and 3 are same as in the above example.
Hence root name is benzenesulfonamide
Step 4: Identify the side chains and indicate their location
It has two side chains.
- Amine group at para position of benzene ring
4-Amino - Heterocyclic ring on amine group of sulfonamide
Amine group at para position of benzene ring
4-Amino
Heterocyclic ring on amine group of sulfonamide
*@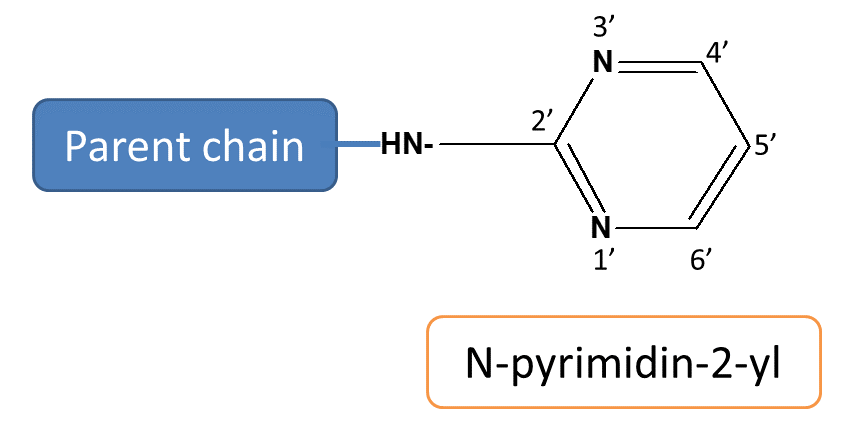
The ring is pyrimidine attached by 2nd position.
Hence N-[pyrimidin-2'-yl]
Step 5:Now arrange the side chains in alphabetical order.
While arranging in alphabetical order, the
4-Amino
N-(pyrimidin-2'-yl)
Step 6 : Complete the name
4-Amino-N-(pyrimidin-2'-yl)benzenesulfonamide
Working Example 4: Sulfacetamide
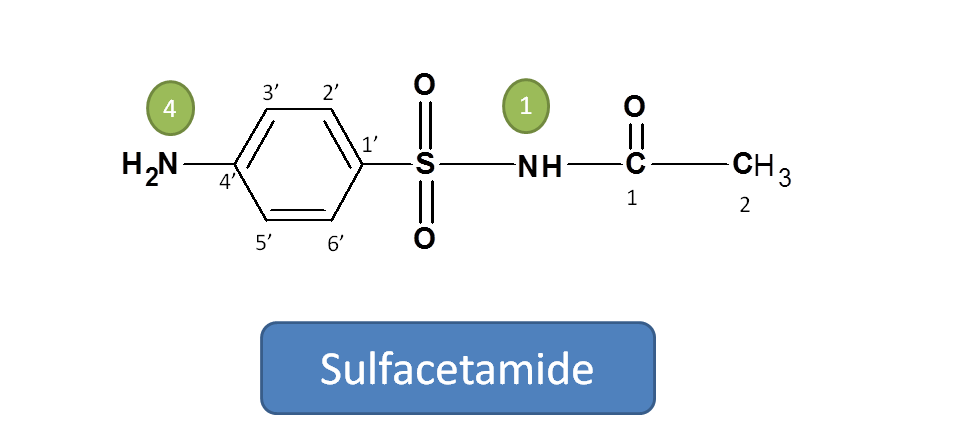
Now let’s take example of sulfacetamide. Even it is pharmacologically similar to other sulfonamides, its IUPAC name varies slightly.
Step 1: Identify the principal functional group
Here two functional groups are present viz. Sulfonamide and acetamide.
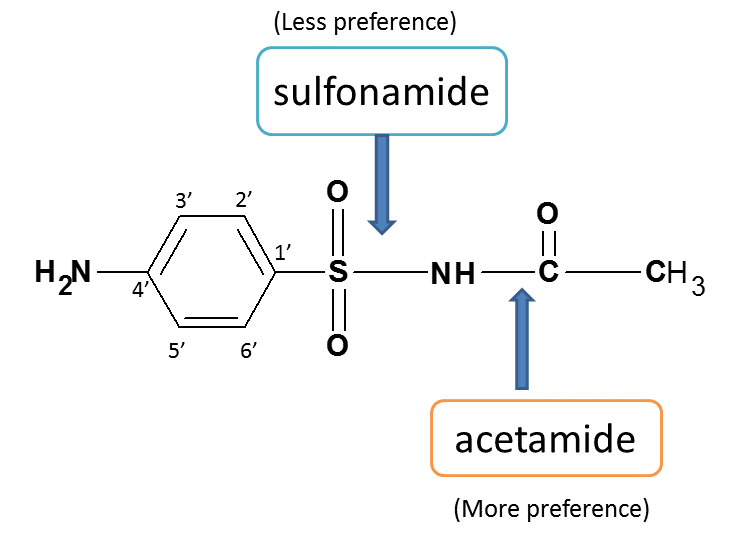
According to IUPAC rules, acetamide should be given more preference and it act as principle functional group here.
Step 2: Identify the parent chain and give the root name
Hence root name is acetamide
Actually, it should be written as ethanamide, but IUPAC allows few of trivial names like acetamide.
Step 3: Give numbering to the structure
Numbering should be started from carbon of amide and parent chain contains two carbons.
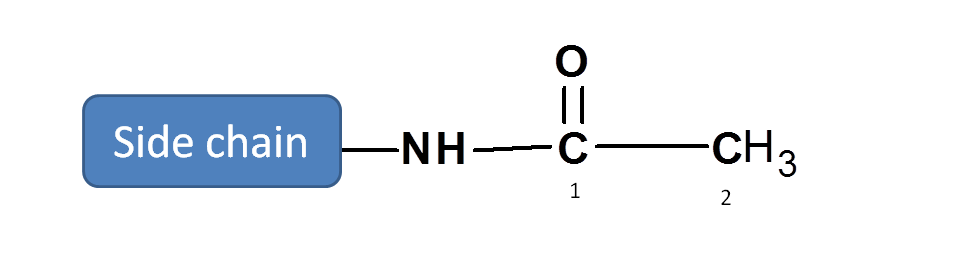
Hence it is acetamide
Step 4: Identify the side chains and indicate their location
Side chain is present at 2nd position of acetamide.
Step 5:Now arrange the side chains in alphabetical order.
Now let’s build the name of the side chain one by one.
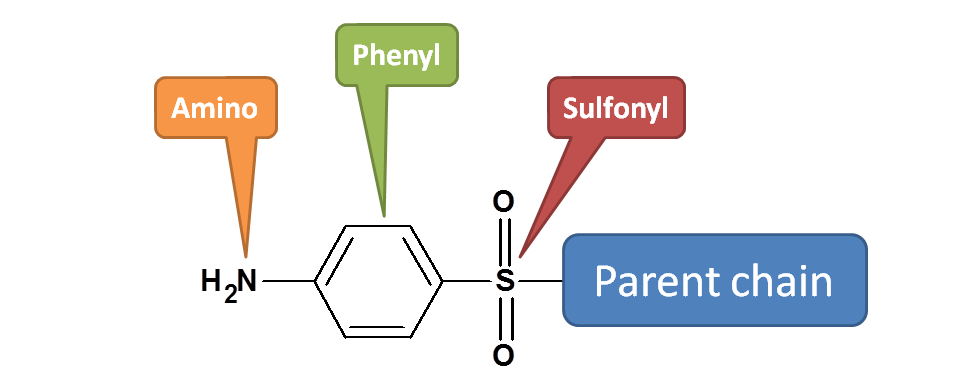
The side chain is immediately attached to main chain by sulphonyl (–SO2-) group. Hence it is
1-[sulphonyl]
Now sulfonyl group is attached to phenyl
1-[(phenyl)sulfonyl]
Phenyl group is attached with amino group at 4th position.
1-[(4-aminophenyl)sulfonyl]
Step 6 : Complete the name
1-[4-aminophenylsulfonyl]acetamide