Aromatic compounds – Who really we are?
by egpat 20 Mar 2019
Aromatic compounds…..Are they produce fragrance always? Of course, very few of them produce aroma but not all. Even though the name is derived from identification of fragrance from these compounds, but not all these aromatic compounds produce fragrance. Then how can you define these aromatic compounds? Simply, by their chemical nature! Yes, these aromatic compounds stand unique in few properties like enhanced stability and their preference to substitution over addition.
It’s great, but how can we identify whether a compound is aromatic or not? Can we say it by just seeing the structure?
Definitely, we can assess a compound whether aromatic or not just by studying few of the criteria for aromaticity in its structure.
For a compound to be aromatic, it should be cyclic, planar and should have conjugated double bonds with odd number of pi bonds.”
❞Let’s take one example here.
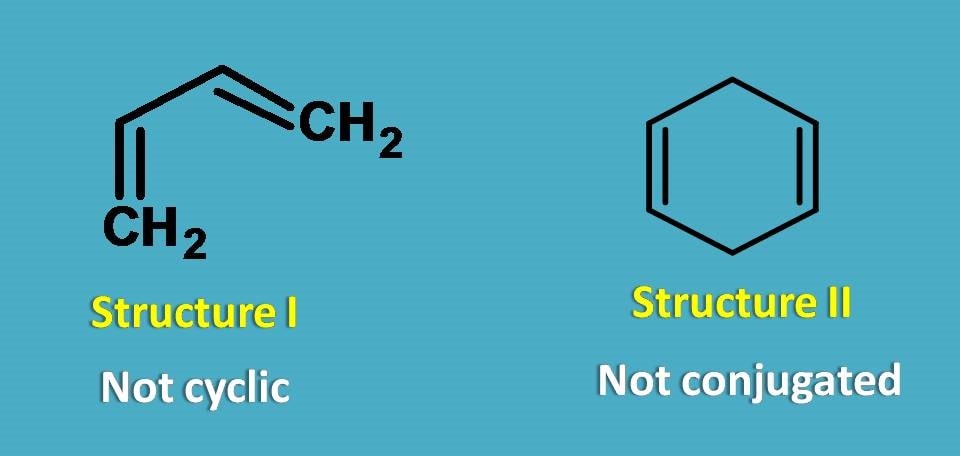
In the above compounds, structure I is not cyclic while structure II is cyclic but doesn’t contain conjugated double bonds. So, both structure I and II are not aromatic compounds.
Now let’s take another example.
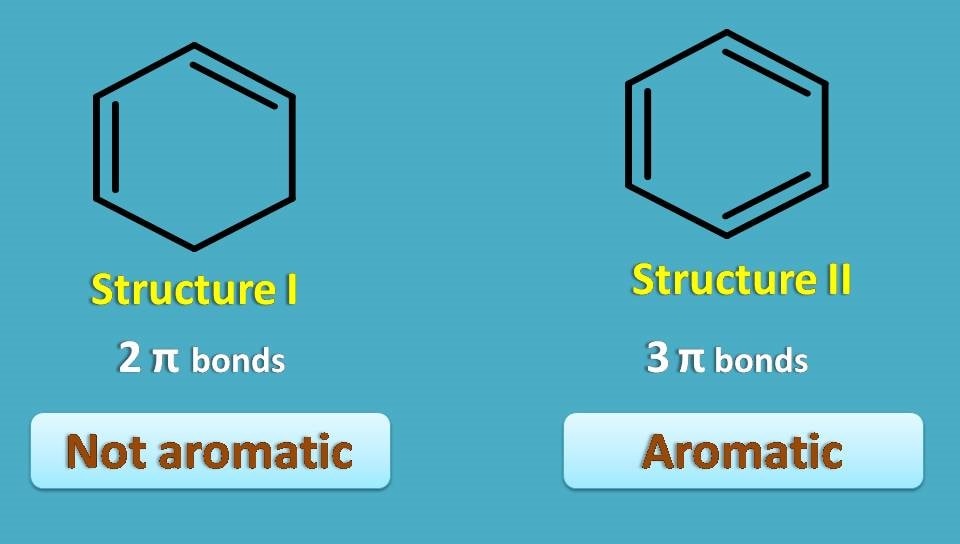
Here structure I is cyclic and contains conjugated double bonds. But it is still not aromatic.
Why? Simply it doesn’t met one of the criteria – odd number of pi bonds.
It contains only two pi bonds hence not aromatic. On the other hand, structure II, well known as benzene, is cyclic, planar, with 3 pairs of conjugated pi bonds hence it is aromatic.
That’s it. Now by finding these criteria in the structure you can easily identify aromatic compounds.
So, let’s go with more examples and find aromatic compounds.
1. Annulenes
As you know that two important criteria for aromaticity are cyclic structure and conjugated double bonds. Annulenes are such type of cyclic compounds with conjugated double bonds with different ring sizes. So it’s good to start our discussion with them and let’s identify which anuulene is aromatic.
Let’s begin.
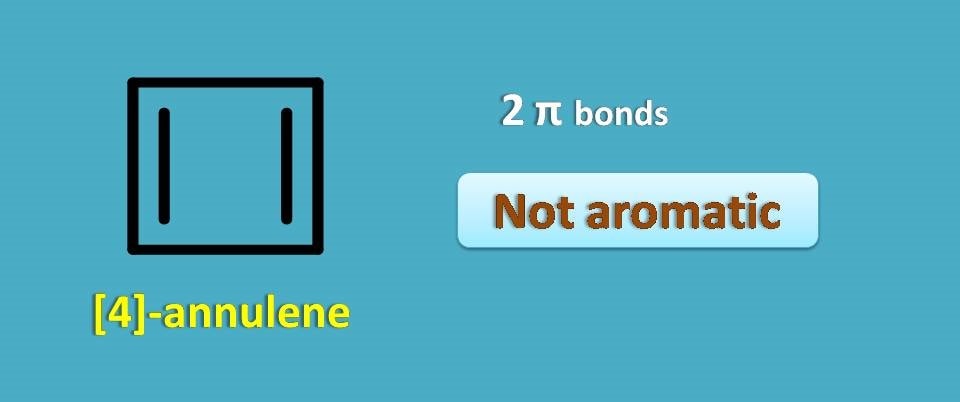
Above structure is a simple annulene with 4 carbons and 2 pi bonds. It is called as [4]-annulene. From the above discussion you can clearly observe that it is not aromatic as it contains even number of pi bonds.
Let’s go with higher annulenes.
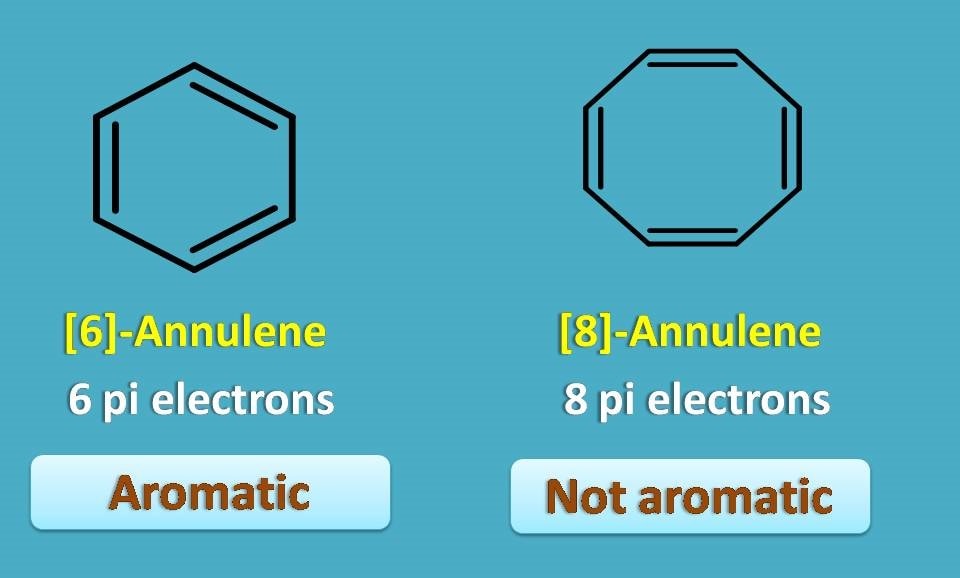
First structure is [6]-annulene which is nothing but benzene and undoubtedly it is aromatic. Second structure is [8]-annulene which again has even number of pi bonds, so not aromatic.
As the annulenes are always cyclic and conjugated, you have to check the total number of pi bonds whether odd or even to assess them to be aromatic or not.
Suppose we represent odd number by 2n+1, an annulene with 2n+1 pi bonds will be aromatic. It can also be stated in another way. As you know each pi bond contains 2 pi electrons, annulene with 2 x (2n+1)=4n+2 pi electrons is again aromatic.
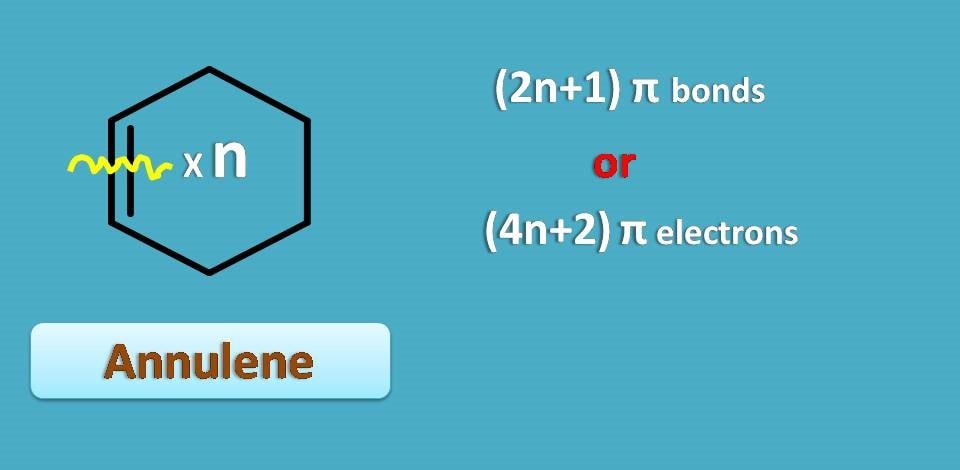
This is somewhat simple as every annulene is prefixed with total number of pi electrons.
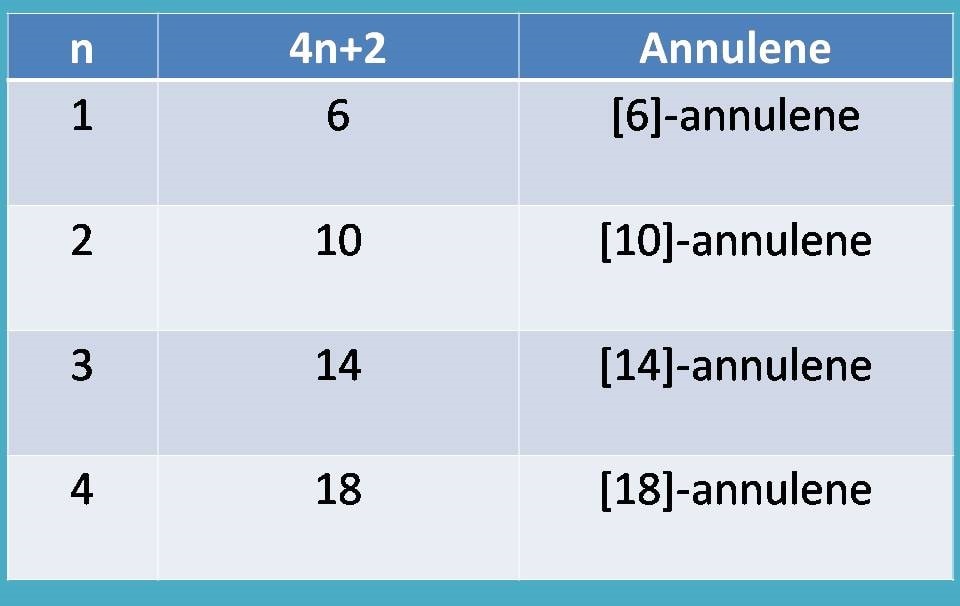
"Therefore, those annulenes with 4n+2 pi electrons are aromatic.”
❞Substituting various values for n=1,2,3,4…..and so on we will get 4n+2 values as 6,10,14,18…..and we can easily assess that they are aromatic.
Wait, the game is not complete yet!!
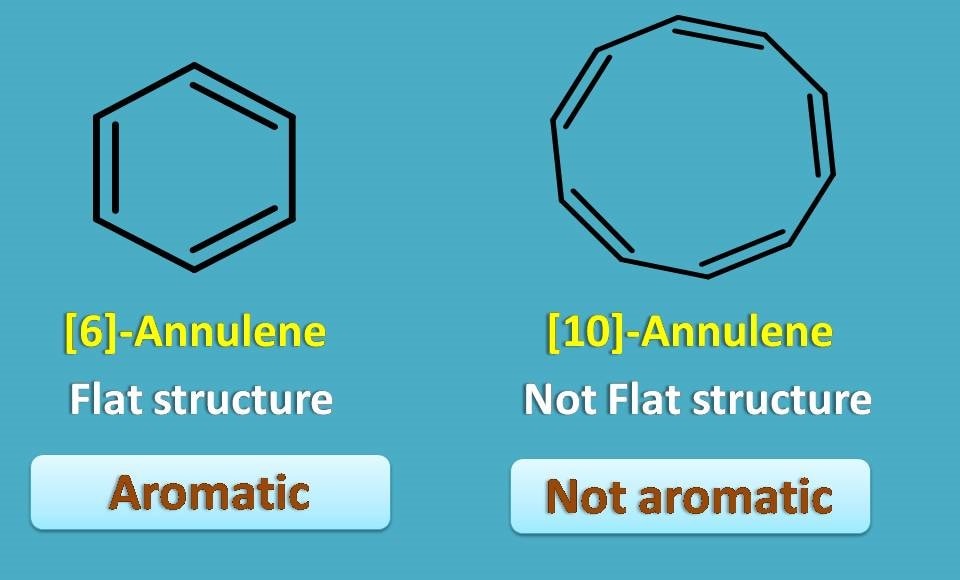
Even most of these are aromatic but not all due to another condition required to be met, that is, structure should be planar.
Even [6]-annulene is flat and aromatic, 10-annulene is not aromatic as it a bent structure due to some ring strain in its conformation.
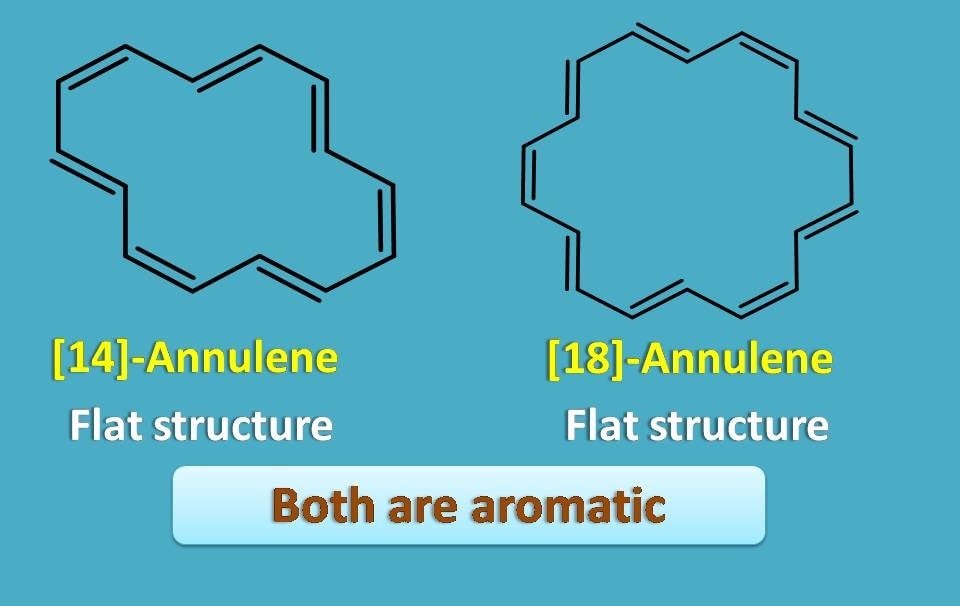
On the other hand, [14] and [18]-annulenes are flat and again aromatic.
2. Polycyclic rings
Till now we have seen monocyclic rings with conjugated double bonds. Now let’s go with polycyclic ring systems and let’s check whether they are aromatic or not.
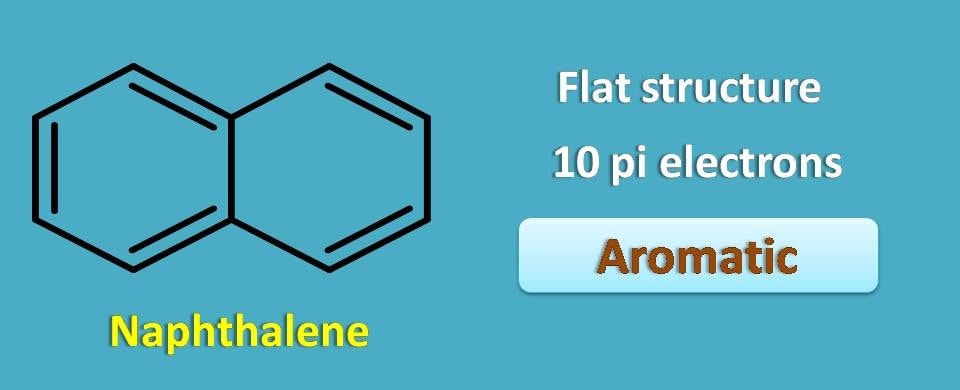
Above structure is naphthalene which is cyclic and planar structure. it contains totally 10 pi electrons with conjugated double bonds hence it is perfectly aromatic.
Similarly the following rings are aromatic as they contain 4n+2 pi electrons.
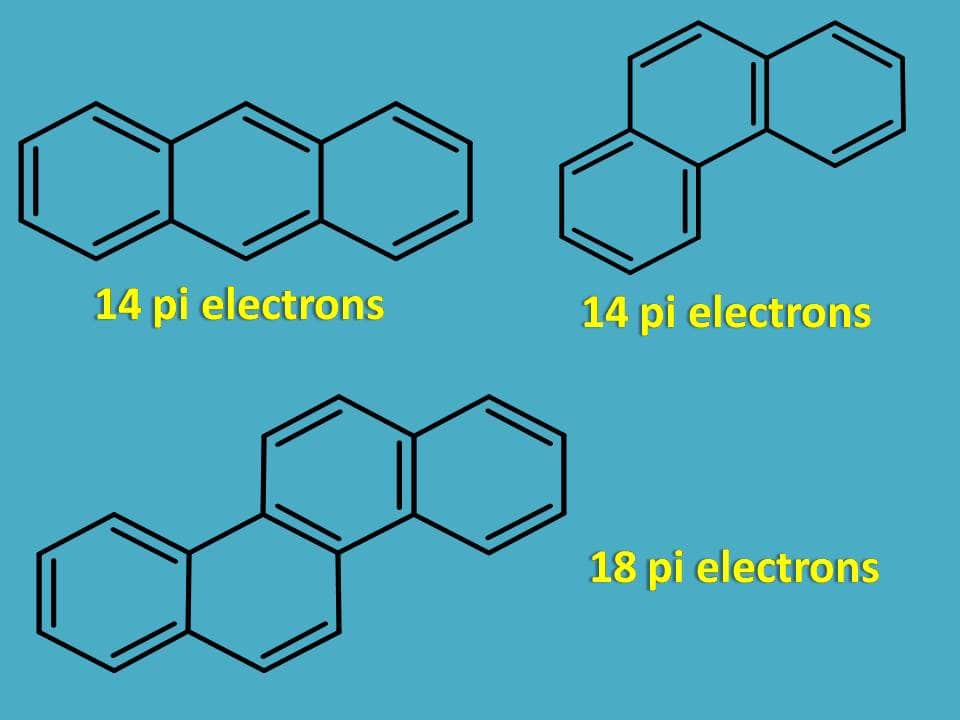
Now let’s see few other examples.
Is it aromatic?
The structure is cyclic, planar and contains conjugated double bonds but the total number of pi electrons is 16 which doesn’t fit with 4n+2 pi rule.
Surprisingly, it is still aromatic!!
Even it has 16 pi electrons only 14 pi electrons participate in the delocalization of electrons in pi cloud above and below the plane bringing aromaticity to the compound.
3. Heterocyclic compounds
Now let’s turn into heterocyclic rings. Are they aromatic?
Let’s take simple example, pyrrole.
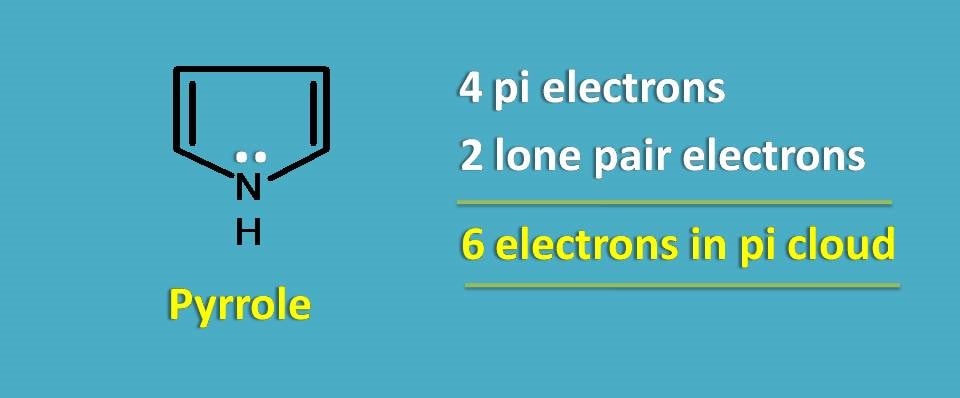
It has only 4 pi electrons, so again it doesn’t fulfil Huckel’s rule. Still the compound is aromatic.
How it’s possible?
It’s all due to lone pair of electrons that contribute to the pi cloud making the total number of electrons participating as 6.
Now let’s take pyridine, six membered heterocyclic ring.
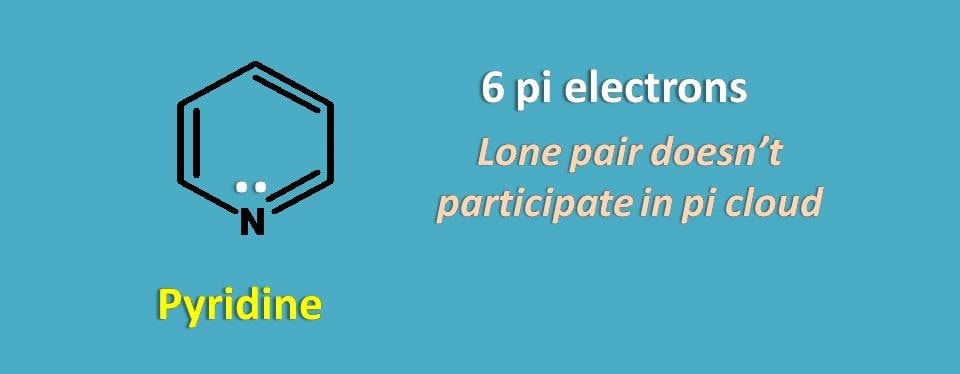
If you count only pi electrons, it contains totally 6 electrons and it obeys Huckel’s rule. But if you consider, lone pair of electrons total electrons are 6 +2=8.
So what happens?
Clearly, the lone pair of electrons will not participate into the pi cloud making effective electrons as 6 in the pi cloud hence aromatic.
In this way, most of the heterocyclic rings attain aromaticity either by including or excluding pi electrons according to the situation arise in the structure.
So, all the following heterocyclic rings are aromatic in nature.
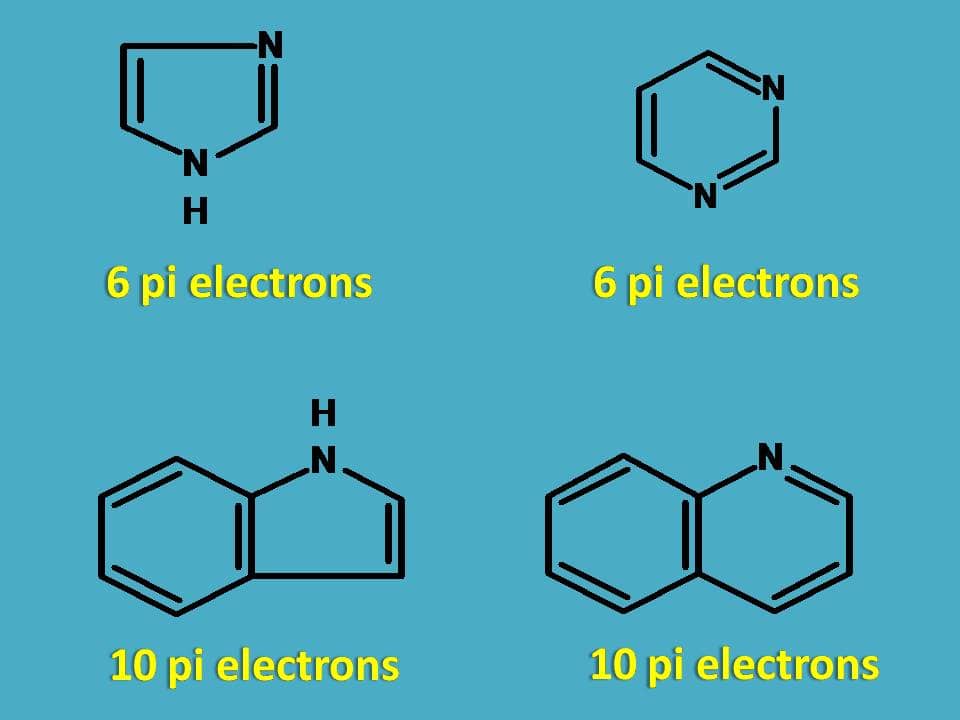
4. Cations and anions
One of the primary role of aromaticity is to delocalize the electrons thereby impart stability to the ring. So, why can’t we apply same logic to the cations or anions that behave as aromatic?
Yes, we will peep into this with simple examples.
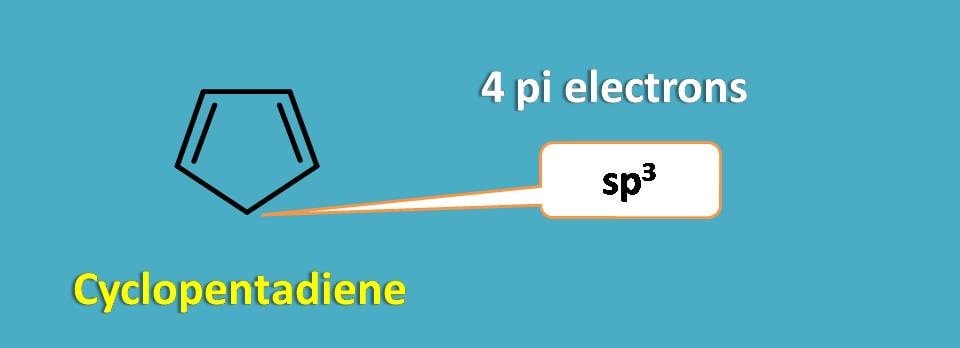
Cyclopentadiene is not aromatic as it contains only 4 pi electrons and even it is not planar as one the of the carbon is sp3 hybridised.
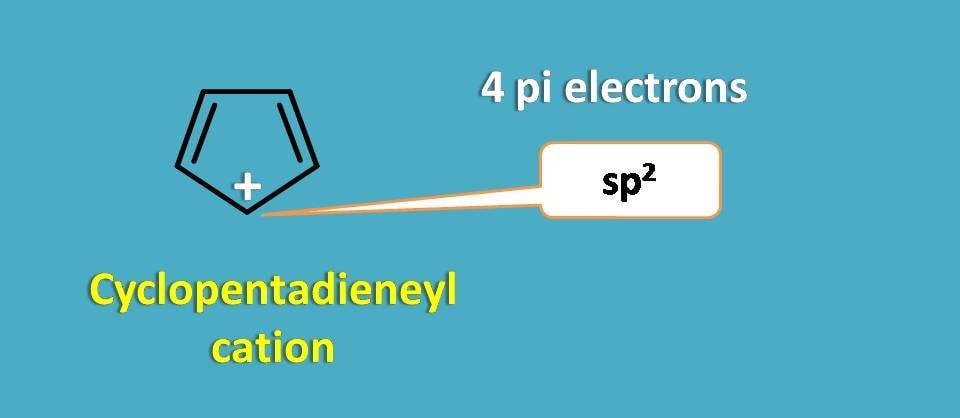
Similarly cyclopentadienyl cation is also not aromatic. Even it is a planar structure due to sp2 hybridization of carbocation, still it contains only 4 pi electrons hence not aromatic.
Now consider cyclopentadienyl anion.
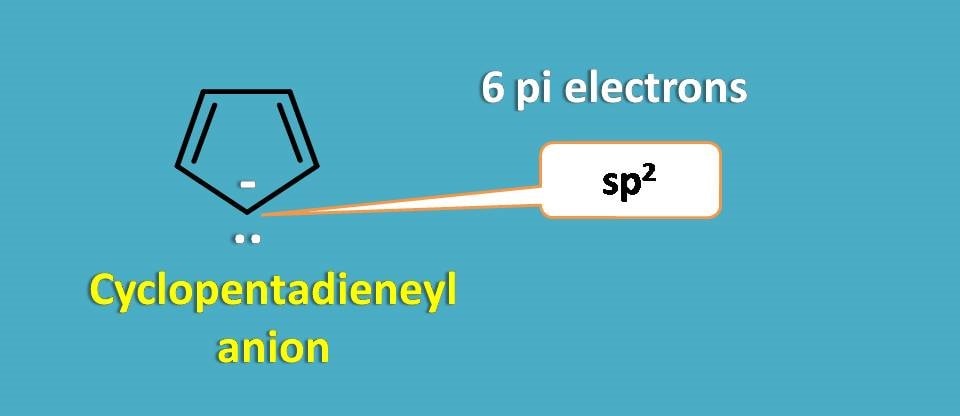
It has extra lone pair of electrons on the negatively charged carbanion, which participates in delocalization and formation of stable pi cloud. Therefore it acts as aromatic compound.
Similarly one of the cation that exhibits extra stability due to aromaticity is tropylium ion.
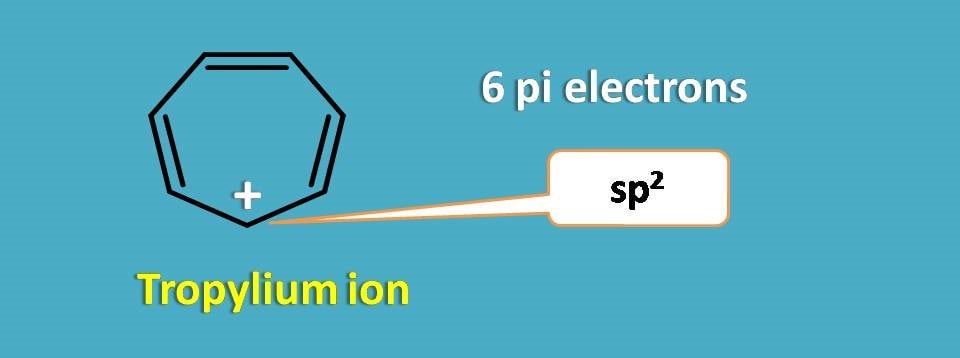
It is a seven membered ring with 6 pi electrons and one positively charged carbocation. Again positive charge on the carbon makes it sp2 hybridised and brings planarity to the structure resulting in aromaticity.
Conclusion
Finally we can conclude that aromaticity is a special property of cyclic compounds with conjugated double bonds that imparts extra stability to ring systems. A cyclic compound, anion or cation always tends to delocalise the electrons and tries to acquire aromaticity. In that process it may include or omit lone pair of electrons to bring a stable aromatic compound. Hope you enjoyed learning about aromatic compounds by reading this article and please share this post with your friends.