Clinically proved prodrugs and their active metabolites
by egpat 03-10-2017
We come across with so many drugs in medical world but all these may not be active and work directly with their target sites. Few of the drugs called prodrugs are inactive forms of drugs and when administered into the body they are metabolized to produce active drug.
How they can be identified?
Prodrugs can be identified by either prefix or suffix to their active metabolite but in few cases they may have completely different names compared to their active metabolites.
Names with prefix
Few of the prodrugs can be named by adding a prefix to their active metabolite. Therefore these drugs can be easily identified.
Prodrug | Active metabolite |
|---|---|
| Valacyclovir | Acyclovir |
| Valganciclovir | Ganciclovir |
| Pivampicillin | Ampicillin |
| Fosamprenavir | Amprenavir |
shoeEGPAT
Names with suffix
Sometimes name of the prodrugs include their active metabolite along with a suffix. Most of these prodrugs are simple salt preparations like phosphates or esters that improve bioavailability of their metabolites. Again these can be easily identified.
For example,
Prodrug | Active metabolite |
|---|---|
| Prednisolone phosphate | Prednisolone |
| Gabapenti enacarbil | Gabapentin |
| Olmesartan medoxomil | Olmesartan |
| Fludarabine phosphate | Fludarabine |
Active metabolites with suffix
In contrast to the above discussion, in few cases, names of the active metabolite can be derived from the name of its prodrug.
For example, ACE inhibitors like enealapril, ramipril, fosinopril and trandopril are prodrugs and the names of their active metbaolites can be obtained by adding suffix “-at” to their names.
Prodrug | Active metabolite |
|---|---|
| Enalapril | Enalaprilat |
| Ramipril | Ramiprilat |
| Fosinorpil | Fosinorpilat |
| Trandopril | Trandoprilat |
Here the suffix “-at” indicates active moiety.
Similarly many of the anticancer antimetabolites are converted into their triphosphate form which is active.
With different names
Finally, few of the prodrugs show little or no relation in their names with their active metabolites.
Prodrug | Active metabolite |
|---|---|
| Azidothymidine | Zidovudine triphosphate |
| Capecetabine | Fluorouracil |
| Famciclovir | Penciclovir |
| Nabumetone | 6-Methoxy naphthalene acetic acid |
| Spironolactone | Canrenone |
| Terfenadine | Fexofenadine |
| Dipivefrin | Epinephrine |
| Omeprazole | Omeprazole sulphonamide |
| Sulfasalazine | 5-Amino salicylic acid |
| Olsalazine | 5-Amino salicylic acid |
| Methanamine | Formaldehyde |
| Bambuterol | Terbutaline |
| Allopurinol | Oxypurinol |
| Simvastatin | Beta hydroxyl acid |
| Tegafur | 5-Fluorouracil |
Why we require prodrugs?
Why we should use prodrugs? Why can’t we use direct active drugs?
Yes, many of the times we use direct active drugs but in few situations we may use prodrugs to
- increase bioavailability
- minimize their side effects.
Increasing bioavailability
Prodrugs can increase the bioavailability of drugs mainly by increasing absorption by various mechanisms
- Increasing aqueous solubility
- Increasing lipophilicity
- Increasing targeted transport
Increasing aqueous solubility
Prodrug concept can be used to increase the aqueous solubility of the active drug. This can achieved by adding a hydrophilic moiety to the drug which improves aqueous solubility hence dissolution. Drugs can be converted into their alkyl esters, phosphates and phsophonates all increasing their solubility.
Amprenavir is such a drug which is a phosphate ester of amprenavir having increased aqueous solubility.
Similarly tenofovir disoproxil is another prodrug prepared as isopropyloxy carbonyloxy methyl derivative of tenofovir with imporved solubility.
Few of the other examples are fludaribine phosphate , estramustine phosphate, and prednisolone phosphate.
Many drugs can be modified into these ester forms to improve solubility and bioavailability parameters. Few of the drugs can aslobe internally esterfied to modify their solutbility.
Note: The prodrug should not compromise the absorption process. Hence it should have good solubility yet highly absorbed through lipophilic membrane. Hence this method is only suitable for drugs having high permeability but poorly aqueous soluble.
Increasing lipophilicity
Prodrugs can also be used to increase lipophilicity of the drugs. In this turn, a lipophilic moiety is added to the parent drug.
For example, pivampicillin is a prordrug of ampicillin that has increased lipophilicity than ampicillin.
Another drug oseltmavir carboxylate also has enhanced lipophilicity.
Increasing targeted transport
For example, acyclovir is an antiviral agent that has poor gastric absorption whereas valacyclovir has enhanced gastric absorption increasing its bioavailability. Valacyclovir is a valine derivative of acyclovir.
Gabapentin is an anticovulsant that shows saturation kinetics of absorption. That means, the absorption increases with dose upto certain extent and after that it shows plateau phase in absorption even the dose is increased. Hence the absorption is saturated. It is somewhat benefit in overdose as it can’t be completely absorbed but in therapeutic point of view it is not desirable. Therefore it can be converted into gabapentin encarbil which is transported by monocarboxylate transpoter across the membrane.
Minimize side effects
Some times prodrugs also eliminate potential toxicity. For example, terfenadine is an antihistamine that can increase QT interval in ECG and can produce potential life threating side effect torsade de pointes. Due to its proarrhythmic effect it was withdrawn from the market. But surprisingly, its prodrug fexofenadine is not associated with any such side effect hence now clinically used.
Prodrugs can also minimize the toxicity and allow their administration in one route where parent drug produces toxicity.
For example, phenytoin is an anticonvulsant that can’t be given by intramuscular (IM) route as it produces tissue necorsis and damage. But its prodrug fosphenytoin can be given by IM route and once it reaches into the blood it is immediately converted into phenytoin.
Sometimes prodrugs can also be invented unknowingly. For instance, the dye pronotsil was found to have antibacterial action but later it was found that it is metabolized to sulfanilamide which is responsible for the activity.
Similarly sulfasalazine is a prodrug of 5-amino salicylic acid found to have anti-inflammatory activity. Sulfasalazine is cleaved by azoreductases into sulapyridine and 5-aminosalicylic acid. Here 5-aminosalicylic acid is the main active metabolite and works as anti-inflammatory agent.
Bioactivation of prodrugs
The main concept of prodrugs is to bring the active drug within a protected environment into the blood where it can be released to act on its target site. So one of the important thing is the release of active metabolite from prodrug.
Many of the ester prodrugs are immediately cleaved to produce active metabolite by simple hydrolysis. These type of prodrugs are designed in such a way that they are stable enough to improve absorption at the same time they can be easily converted into active metabolite in-vivo.
Few of the prodrugs can undergo small biomolcular modification to produce active metabolite. Sulindac is a non-steroidal anti-inflammatory agent that is inactive and converted into active metabolite. It undergoes phase I metabolism where it simply undergoes a change in oxidation state of sulfur.
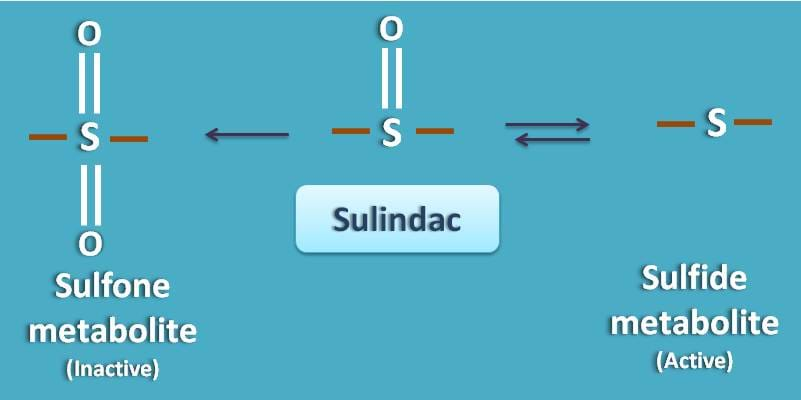
It produces two metabolites such as sulfone and sulfide metabolite. The sulfide metabolite is active.
Similarly many of the drugs are originally prodrugs that release an active moiety in-vivo by biomolecular modification. Examples include
Prodrug | Active metabolite |
|---|---|
| Omeprazole | Omeprazole sulphonamide |
| Fenofibrate | Fenofibric acid |
| Spironolactone | Canrenone |
| Sulfasalazine | 5-Amino salicylic acid |
| Simvastatin | β- hydroxyl acid |
| Clopidogrel | Thiol metabolite |
| Allopurinol | Oxypurinol |
| Mycophenolate mofetil | Mycophenoilc acid |
| Nabumetone | 6-Methoxy naphthalene acetic acid |
| Latanoprost | Latanoprost acid |
Are they slow acting?
No. Even they require bioactivation this doesn’t decrease their onset of action as all the drugs should undergo metabolism rapidly before they reach their target organ.
Can we make prodrug for all types of drugs?
Even prodrugs have so many advantages, they are not suitable for all drugs. A prodrug should have following characters.
- It should be chemically stable
- It should have good absorption
- It should release active drug easily
- It should not be expensive
Most of the times, preparing prodrugs is an expensive process hence should be used only in required situations.
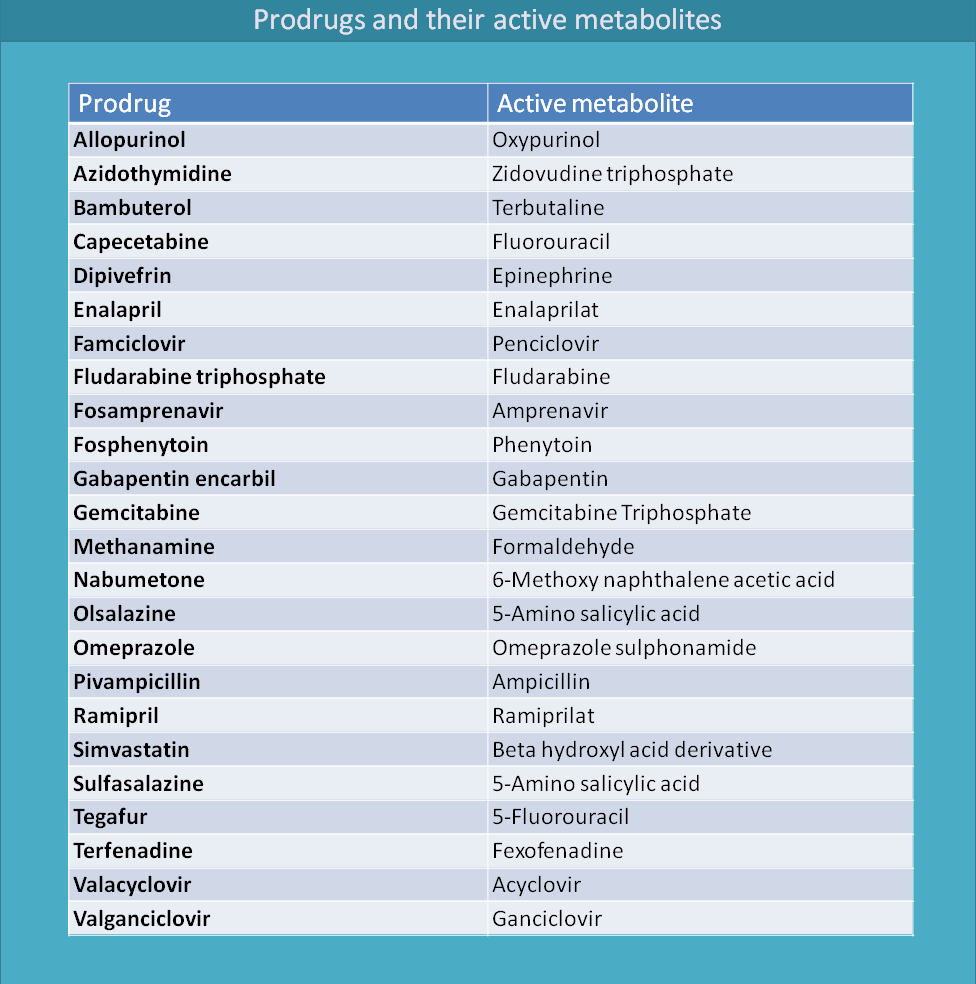
Here is the summary of many prodrugs and their metabolites.