How to write IUPAC name – practical examples
by egpat 19 Mar 2019
How wonderful it will be when you able to write IUPAC name of a given compound without any ambiguity. You can learn new structures very easily when you know their names even you can remember and analyse well. For all that you require an ample practice with thought-provoking examples. Here we will see such practical examples and this exercise clearly flush outs any doubts in your mind and makes you feel confident in naming organic compounds.
Let’s start with simple structures.
IUPAC practical example 1:
Consider the following structure.
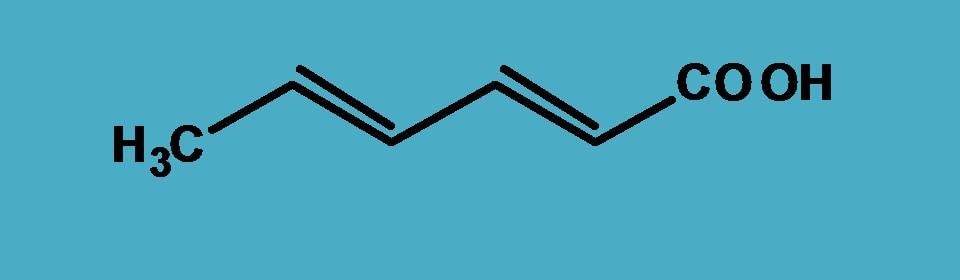
First, we have to identify principal functional group clearly here it is carboxylic acid (-COOH) according to the priority order . Hence the primary suffix is “-oic acid”.
Now let’s start the numbering from the carboxylic acid as it should be given least possible number.
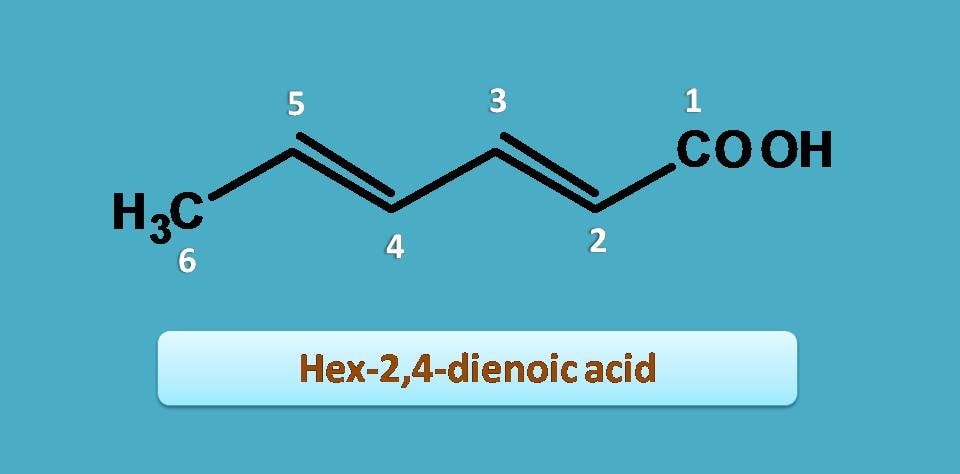
Since double bonds are present at 2nd and 4th positions, another suffix is “-diene”.
Combining all these,
Hex + 2,4-diene + oic acid=Hex-2,4-dienoic acid
IUPAC practical example 2::
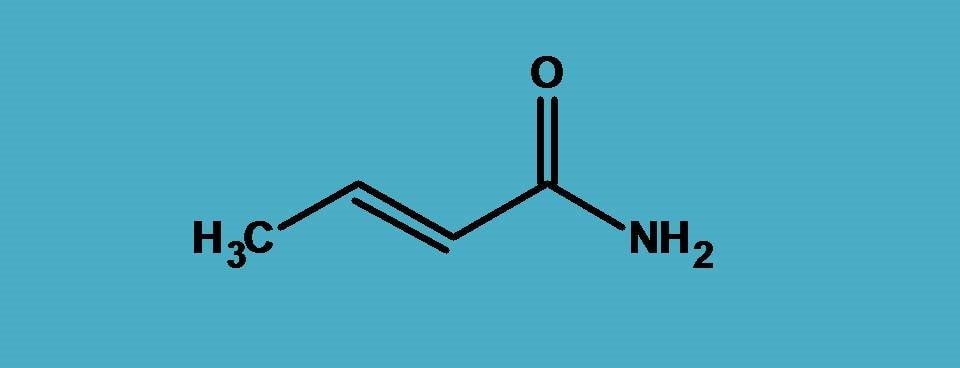
In the above example, amide –CONH2, acts as principal functional group therefore suffix is “-amide”.
If you count the carbons, totally the parent chain contains four carbons hence the root name is ‘’But-”. Let’s start the numbering. As you already familiar, least number should be given to the principal functional group. So we have to start numbering from carbon of the amide group.
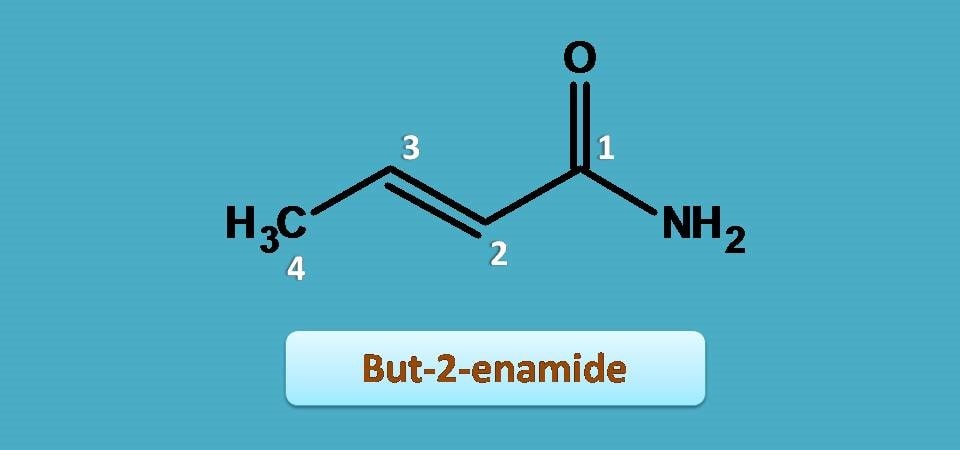
Now, you can find a double bond ar 2nd position, so we have to indicate it by “2-ene”.
Let’s assemble all these fragments to build the name of the given compound.
But + 2-ene + amide= But-2-enamide
It can also be called simply as 2-butenamide.
IUPAC practical example 3:
Let’s consider the following example.
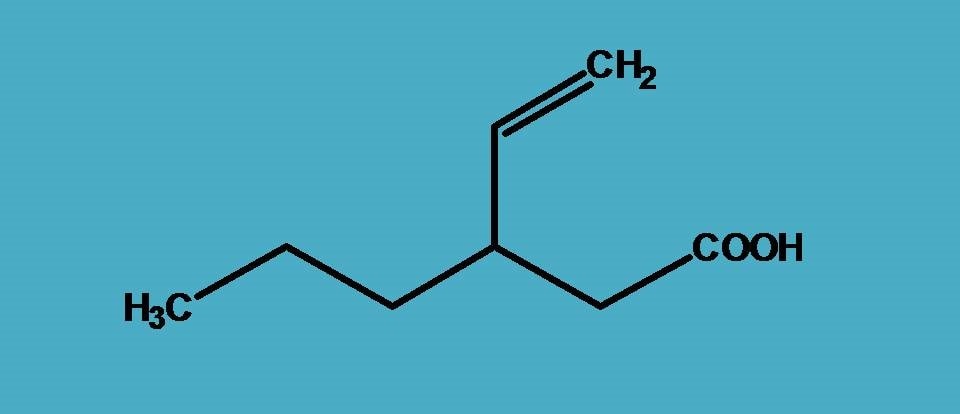
The structure contains carboxylic acid and an alkene functional group and obviously from the previous example, you already familiar that carboxylic acid should be selected as principal functional group hence suffix is “-oic acid”.
Next part is to select longest chain. Here two possibilities arise. One with six carbons and one functional group while other with five carbons yet two functional groups such as carboxylic acid and alkene. When you have a situation like this, you should always stick to the longest chain rule, that is, you have to select the longest chain possible that includes principal functional group.
So, here the six carbon chain is the parent chain hence root name is “hex-”.
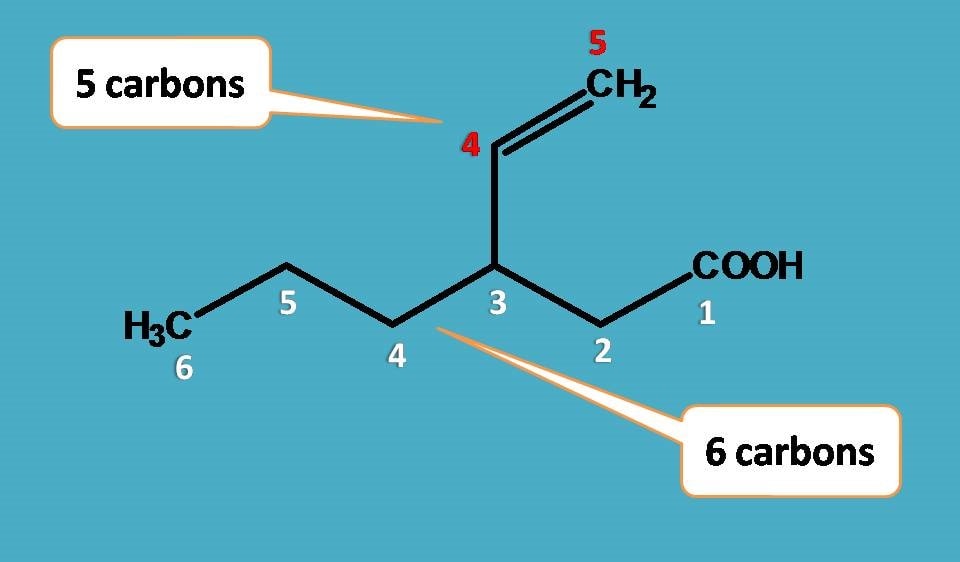
Now let’s see the naming of side chain. Remember that we have to start the numbering of the side chain from the point of attachment. So, the side chain contains two carbons with double bond at 1st position. Hence the name of the side chain is
Eth + 1-ene +yl=Eth-1-enyl
That’s perfect! What else?
Where this side chain is attached? Clearly it is at 3rd position. So, arranging them all, name of the given compound is
3-(Eth-1-enyl)hexanoic acid
Name of the side chain can also be commonly called as vinyl, therefore it can also be written as
3-vinylhexanoic acid.
IUPAC practical example 4:
Let’s take another example.
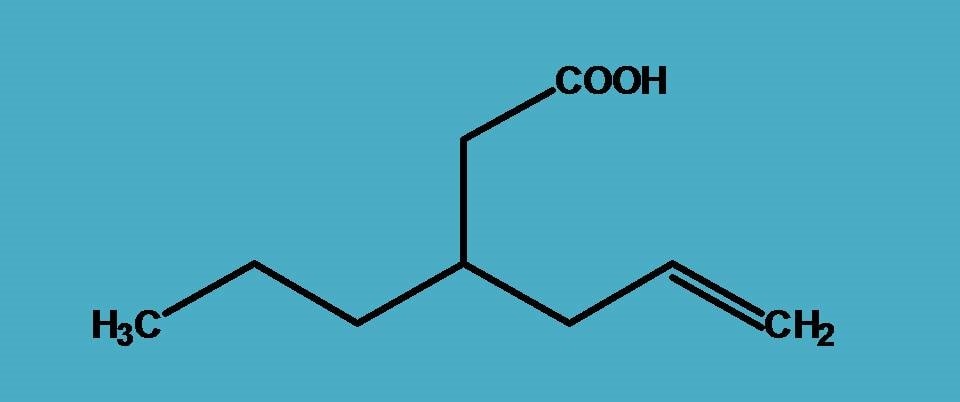
Here again, carboxylic acid is the principal functional group and suffix is “-oic acid”. But here more than two chains are possible and of these two you can find a longest chain with seven carbons and another chain with six carbons. Which has to be selected, seven carbon longest chain?
Certainly, it is not the parent chain here and we have to select six carbon chain.
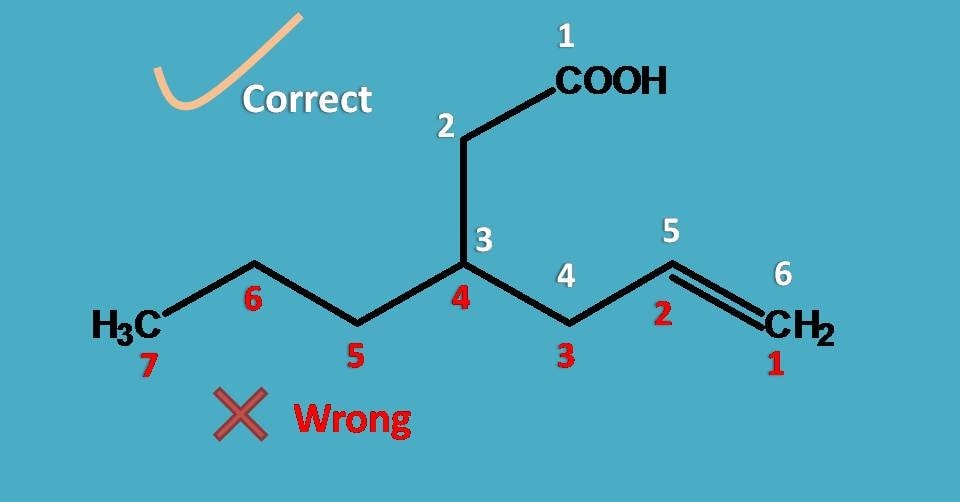
Aren’t we violating longest chain rule? In the previous example we have clearly stressed that parent chain should be longest but here we have done something different. Is it correct?
Yes, we are correct! We still agree that parent chain should be longest, but at the same time it should include principal functional group.
Here chain with seven carbons is longest but doesn’t include carboxylic acid hence it is not the parent chain while the chain with six carbons includes carboxylic acid hence it is the parent chain. Therefore the root name is “Hex-”.
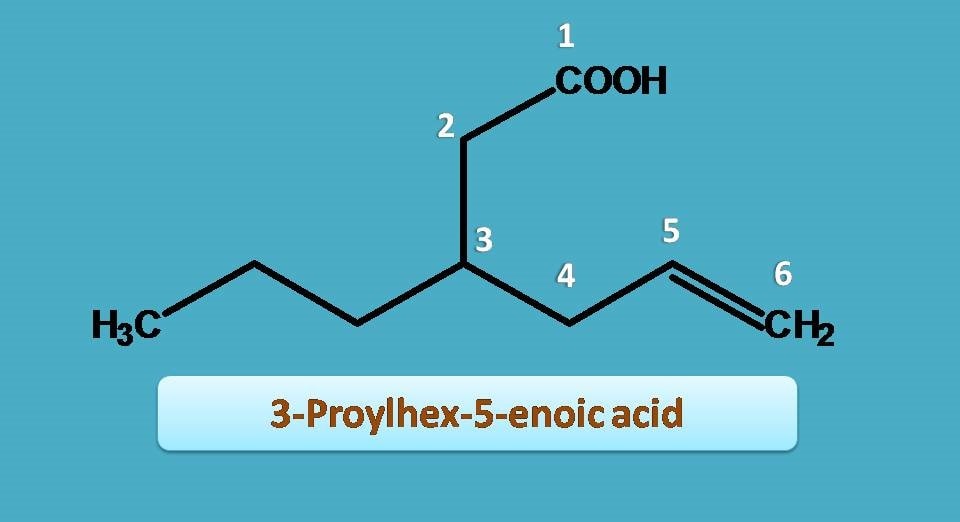
Now, a three carbon side chain is present at 3rd position hence it is 3-propyl and double bond is present at 5th position hence “5-ene”.
Arranging them all, name of the compound is
3-Propyl + Hex + 5-ene + oic acid=3-Propylhex-5-enoic acid
IUPAC practical example 5:
Yes, here is an example now with two functional groups, one is carboxylic acid and another is hydrazide. You should not confuse latter with amide which is –CONH2.
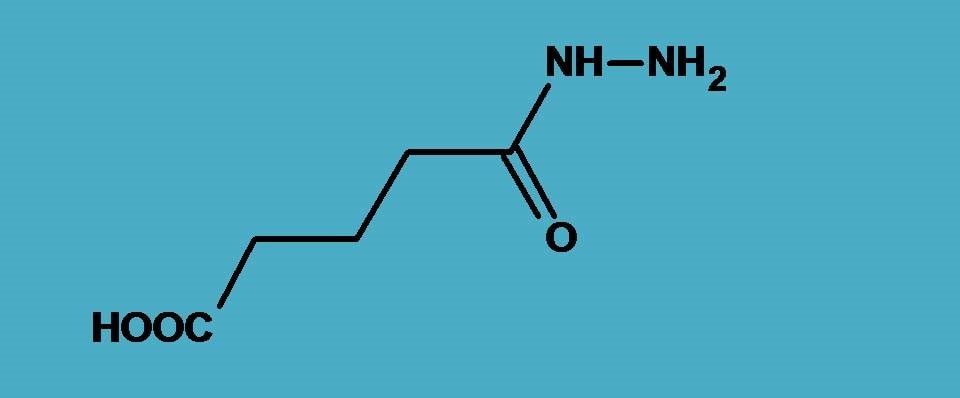
That’s fine. Now, which should be selected as principal functional group? According to the priority order of functional groups, carboxylic acid should be selected as principal group. So the right suffix that we have to use is “-oic acid”.
Next step is to select longest chain. Since only one chain is present, we can select longest chain containing five carbons. Surprisingly, the carbonyl group is also counted even though it is the part of the hydrazide. Suppose if you don’t include this carbonyl group you will get a chain with four carbons which is against to the longest chain rule.
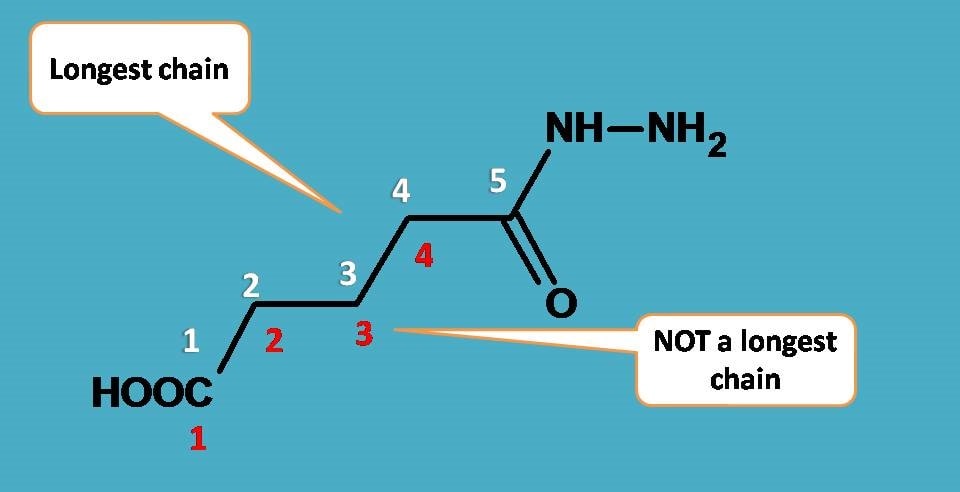
Therefore the parent chain contains five carbons hence root name is “pentan”.
Finally, let’s identify the substituents. At 5th position carbonyl group is present hence indicated as 5-oxo and hydrazine is attached to 5th position hence 5-hydrazino.
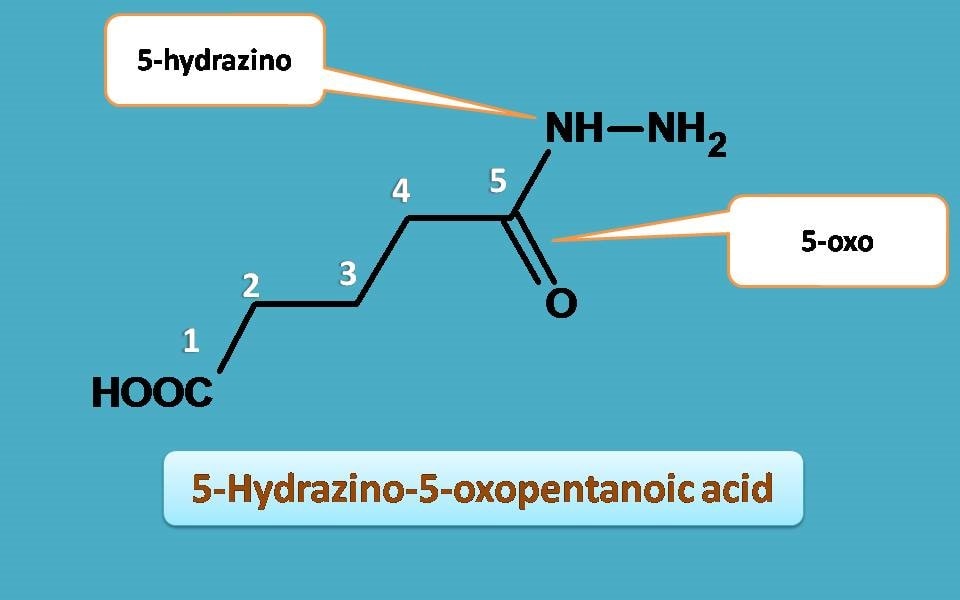
Combining all at one place, name of the compound is
5-Hydrazino-5-oxopentanoic acid
Note: Even hydrazide is the functional group present herer, it is not considered as such when it acts as substituent. So it is splitted as hydrazide=carbonyl + hydrazine. You should not the difference in suffix “-zide” indicates with carbonyl group while “-zine” indicates without carbonyl group.
In this way, we can see write IUPAC names of man compounds with simple set of IUPAC rules . Hope you have enjoyed the article and we will add more number of examples for writing IUPAC names in near future. Don’t forget to this article with your friends and stay tuned to our blog for upcoming posts.