IUPAC nomenclature of benzodiazepines
by egpat 02-10-2017
Benzodiazepine
If we examine the name and split it into three parts, it will be combination of benzo + diaza + epine.
The letter “a” from “diaza”is removed during conversion into single word.
The first term “Benzo” indicates it is a fused ring system with benzene. The middle part “diaza” indicates the ring has two nitrogens. The last part “-epine” indicates it is a seven numbered ring containing nitrogen.
So it is a fused ring system of benzene and diazepine.
Numbering in benzodiazepines
According to IUPAC we have to follow few of the rules in numbering and naming of fused ring systems. Let’s explore one by one.
1. When a heterocyclic ring is fused with benzene, it is indicated by the prefix “benzo”.
Obviously we can find this prefix in their names as we call them as benzo + diazepine=benzodiazepine
2. The numbering to the fused ring system should be started from immediate atom adjacent to fusion atom.
Fusion atoms are also called as bridge heads. These are the common atoms for both the rings which are involved in the fusion of rings.
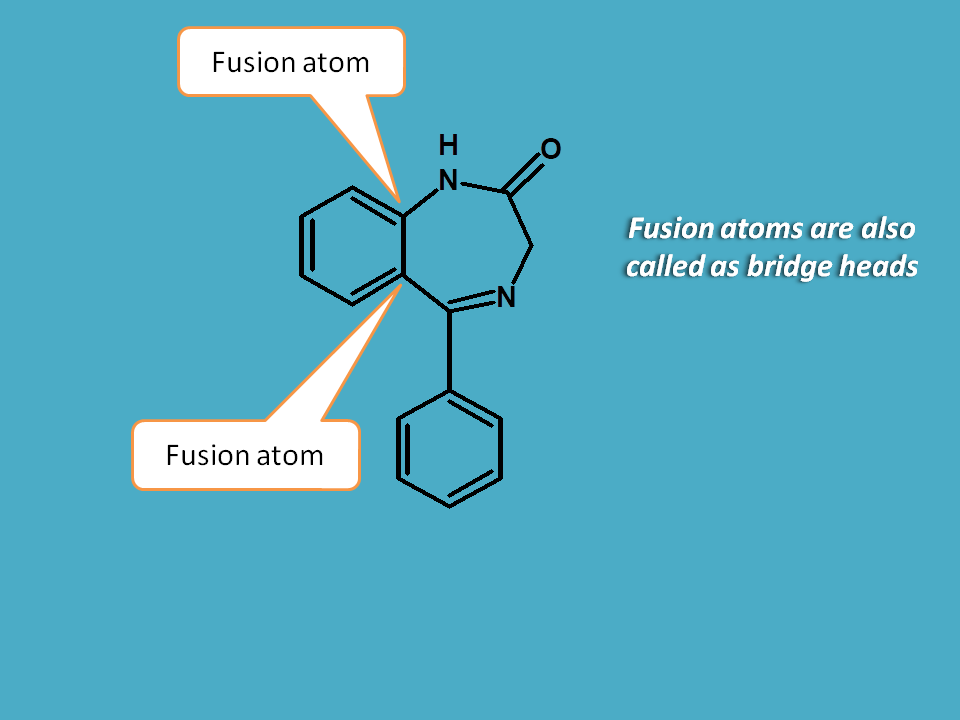
Since benzodiazepine has two fusion atoms, it can have two possibilities for numbering.
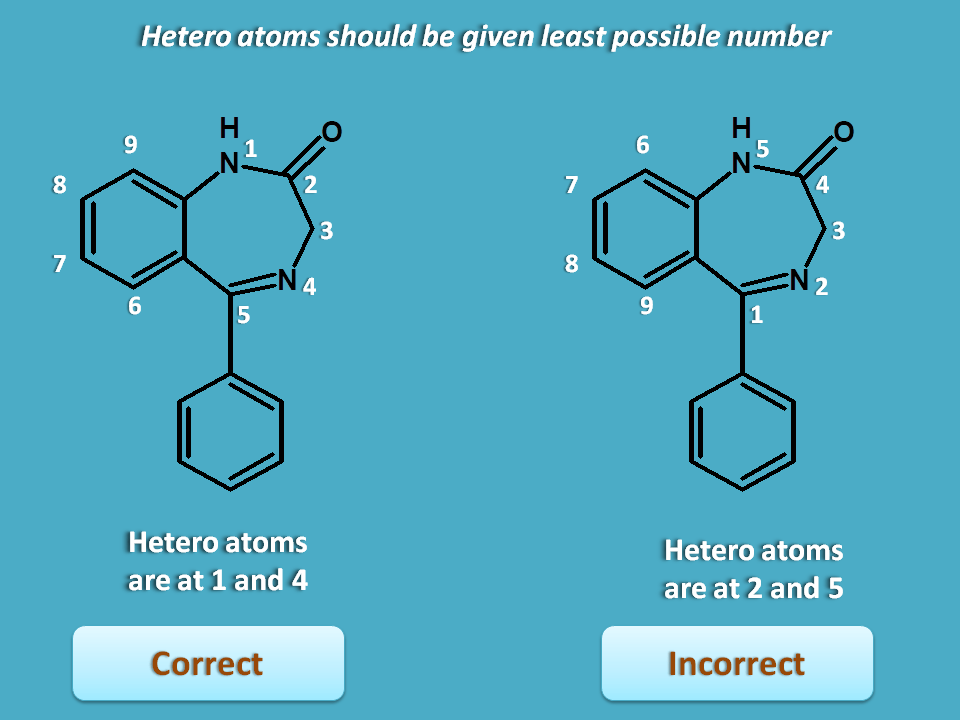
Here clockwise direction has given 1,4 to the hetero atoms while other direction gives 2, 5. Since least possible number should be given to heteroatom, the first direction is correct.
3. Bridge atom or fusion atoms are not numbered.
You can clearly observe here that bridge atoms are not given any number.
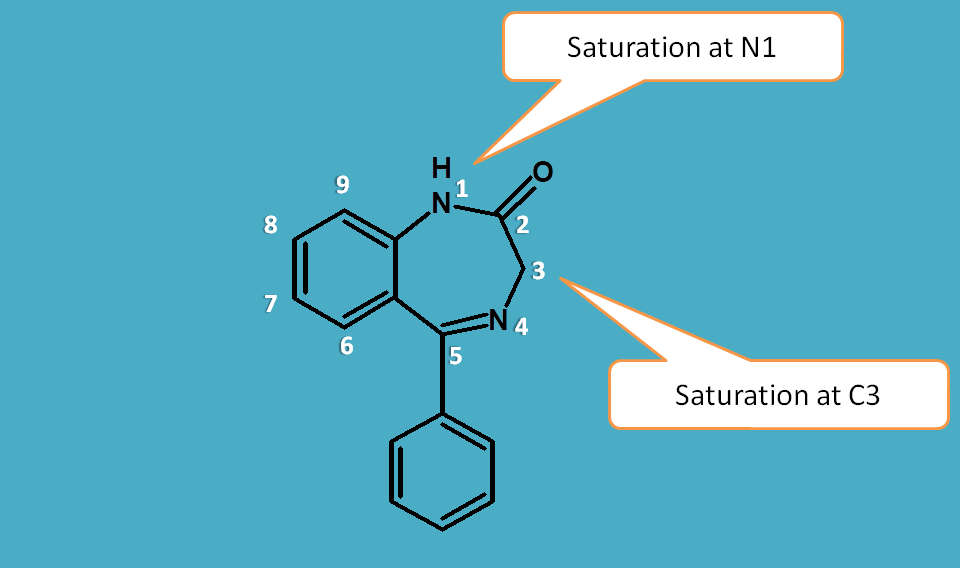
Indicated hydrogens
In heterocyclic rings the point of saturation may have two or more possibilities.
For example, indole can be structurally represented in two ways. In the first structure saturation is present at 1st position whereas in second structure saturation is at 3rd position.
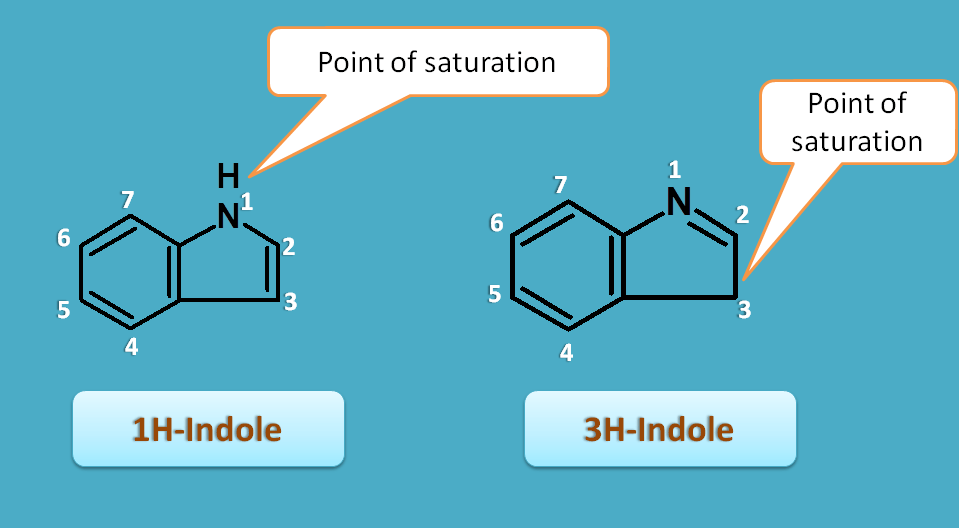
In such situations the point of saturation is indicated by letter “H” prefixed by its location. Hence the first structure is indicated as 1H-indole while second one as 3H-indole.
According to the IUPAC, these hydrogens are called as “extra hydrogens” as they are now in excess when there is a double bond previously at that position.
When saturation is present at two positions, it can be indicated by prefix “dihydro”.
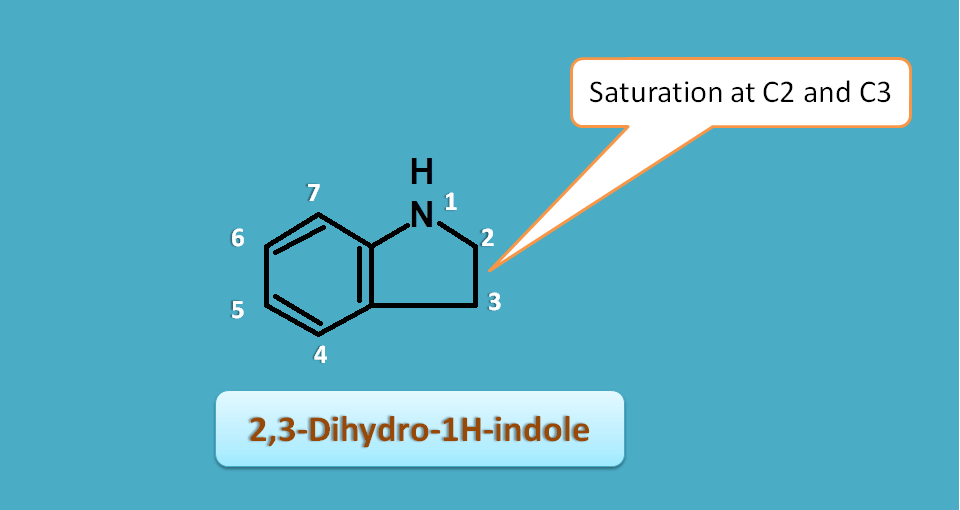
For example if the 1H-indole is saturated at 2nd and 3rd position , it can be named as 2,3-dihydro-1H-indole.
Similarly in case of introduction of keto group, extra hydrogen at adjacent or at other atoms can be indicated by “dihydro” along with their location.
Here in the benzodiazepine ring, keto group is present at 2nd position and extra hydrogens are present at 1st and 3 rdposition hence indicated by 1,3-dihydro.
So the name of this basic ring is
1,3-dihydro-1,4-benzodiazepin-2-one
At second position, keto group is present where actually two hydrogens, in which one being extra hydrogen, are present. Hence it can be indicated by 2H and the basic name will be
1,3-dihydro-2H-1,4-benzodiazepin-2-one
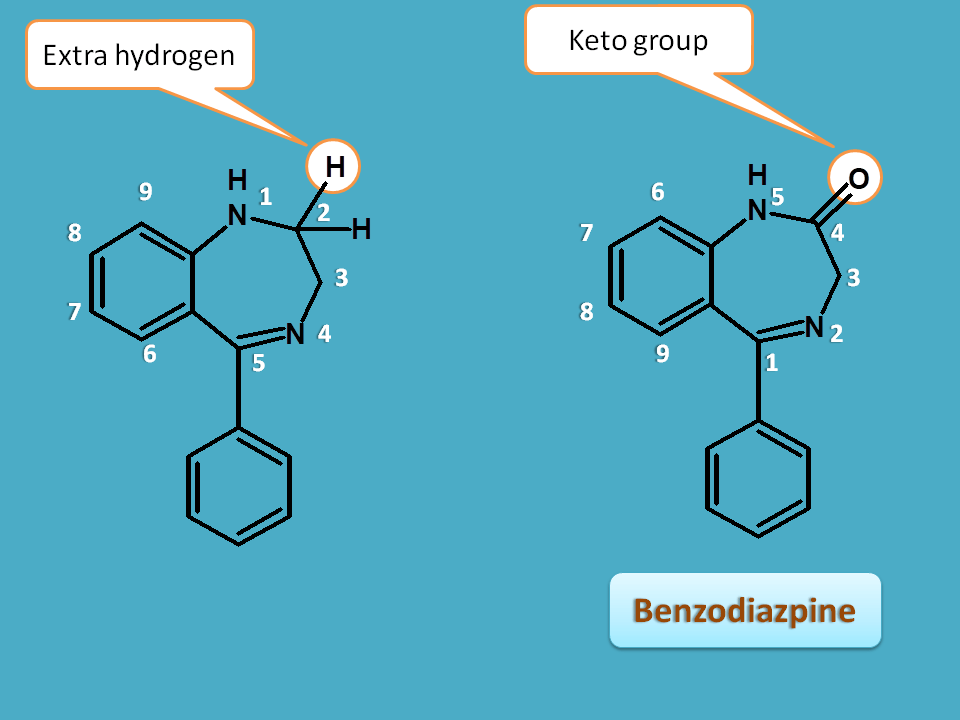
But actually IUPAC prefers first representation so that 2H can be omitted.
IUPAC names
Many of the names of benzodiazepines follow the above IUPAC basic name with little modifications. So let’s see the structures of few benzodiazepines along with their names.
Diazepam
Diazepam contains all the structural features required for a benzodiazepine.
- Keto group at 2nd position
- Electron withdrawing group at 7th position
- Small alkyl group at 1st position
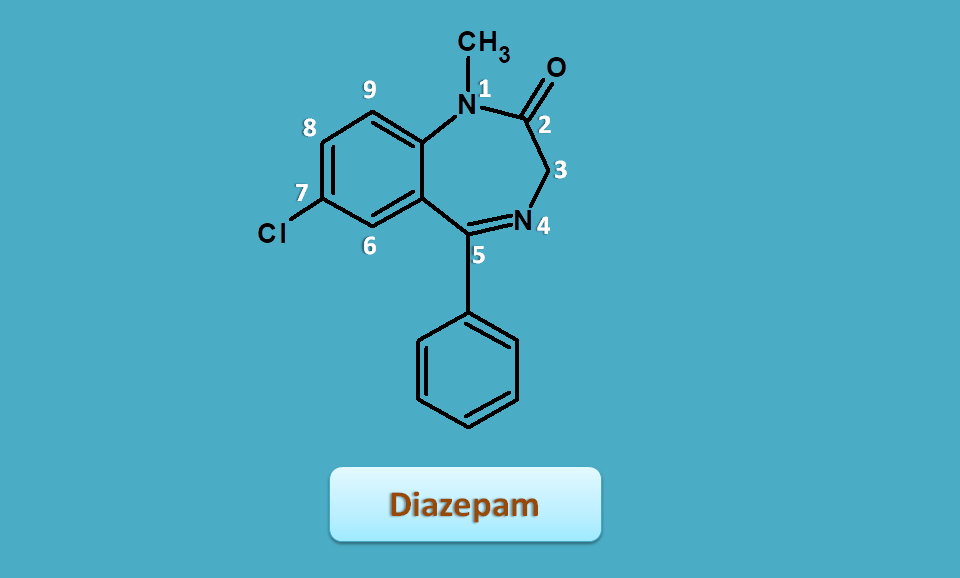
Now if we observe for extra hydrogens, only one extra hydrogen is present at 3rd position hence it can be indicated by "3H". Keto group is present at 2nd position, therefore the basic name is
3H-1,4-benzodiazepin-2-one
Side chains are present at various locations as
- 1-methyl
- 5-phenyl
- 7-chloro
Therefore arranging these prefix in alphabetical order, the IUPAC name pof diazepam is
7-choloro-1-methyl-5-phenyl-3H-1,4-benzodiazepin-2-one
The extra hydrogens can also be represented in another way. Originally they are present at 1 and 3rd positions where hydrogen at 1st position is replaced by methyl group. Hence it can be written as
1,3-dihydro-1-methyl
Similarly, keto group is present at 2nd position, where originally extra hydrogen is present. Hence
1,3-dihydro-1-methyl-2H
So, complete name is
7-choloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one
IUPAC prefers first representation, hence at rest of structures we go with IUPAC name.
Oxazepam
It also shares similar structural features with hydroxy group at 3rd position.
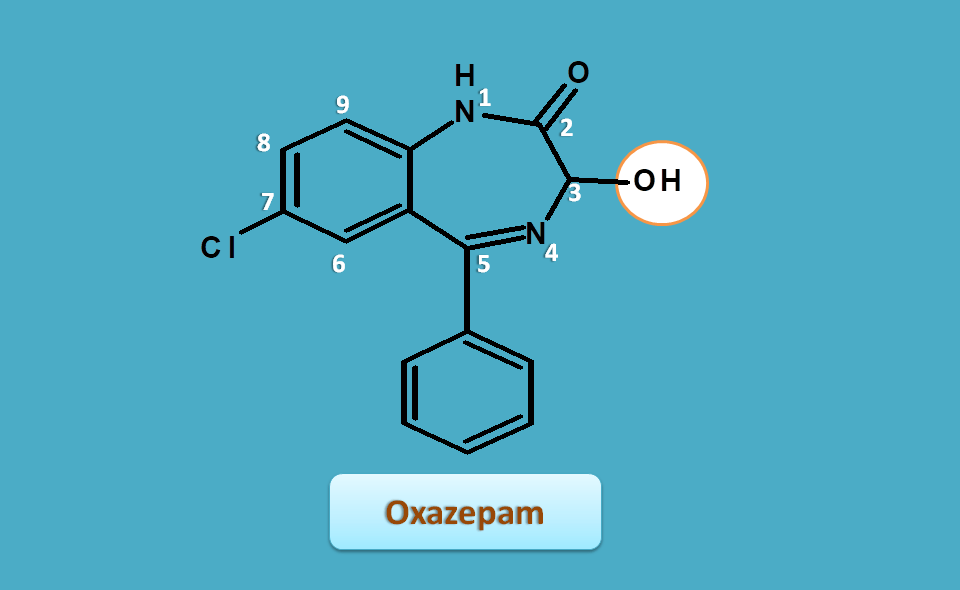
Extra hydrogen are present at 1st and 3rd positions, hence the base name is
1,3-dihydro-1,4-benzodiazepin-2-one
Side chains are present as
- 3-hydroxy
- 5-phenyl
- 7-chloro
Hence the IUPAC name of the oxazepam is
7-choloro-3-hydroxy-5-phenyl-1,3-dihydro-1,4-benzodiazepin-2-one
Lorazepam
This is just similar to oxazepam with extra chlorine at ortho position of 5-phenyl ring.
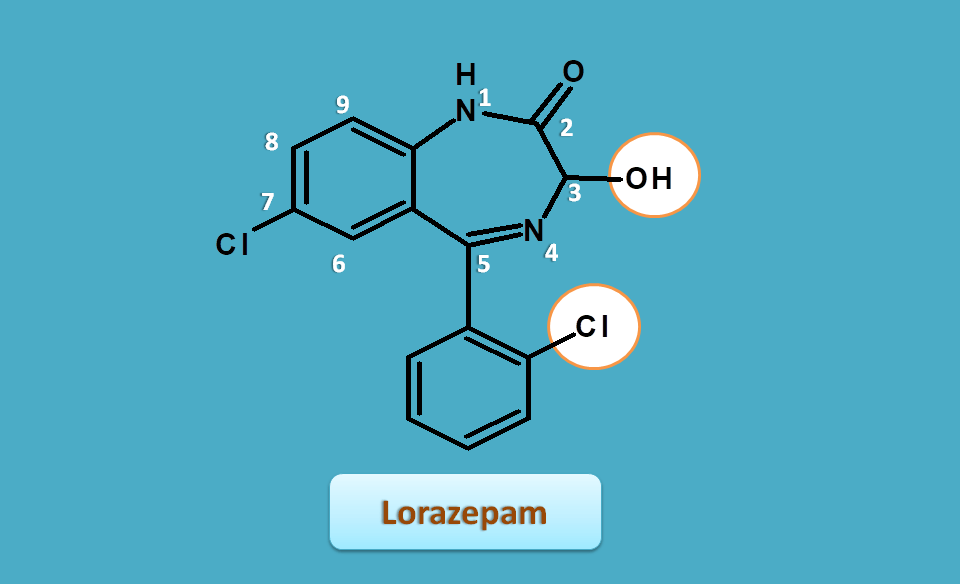
So the base name is
1,3-dihydro-1,4-benzodiazepin-2-one
Side chains are present as
- 3-hydroxy
- 5-(2-chloro)phenyl
- 7-chloro
Henc the IUPAC name of the Lorazepam is
7-choloro-3-hydroxy-5-(2-chloro)phenyl-1,3-dihydro-1,4-benzodiazepin-2-one
Temazepam
Temazepam is again similar to diazepam with a hydroxyl group at 3rd position.
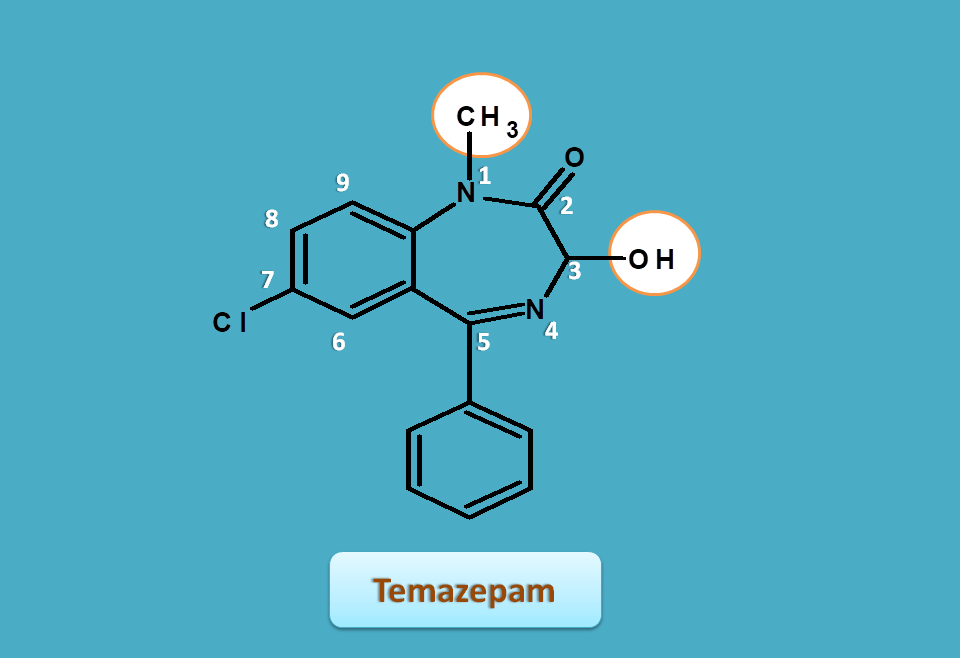
You can compare it with diazepam, hence the base name is
3H-1,4-benzodiazepin-2-one
Side chains are present at
- 1-methyl
- 3-hydroxy
- 5-phenyl
- 7-chloro
Therefore the IUPAC name of temazepam is
7-choloro-3-hydroxy-1-methyl-5-phenyl-3H-1,4-benzodiazepin-2-one
Nitrazepam
Nitrazepam is one of the benzodiazepines that has nitro group instead of chloro at 7th position. Since nitro group is more electron withdrawing nitrazepam is more potent.
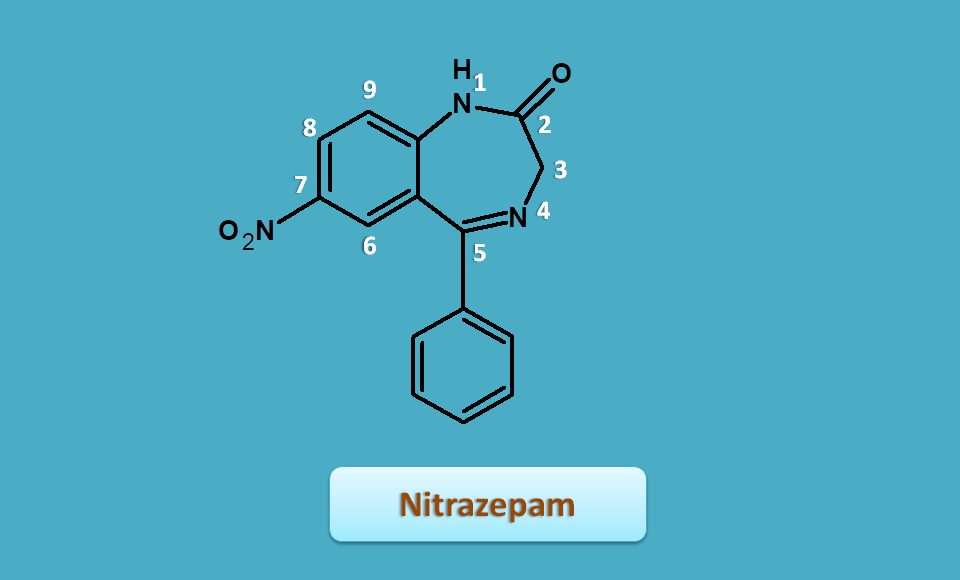
Since it has extra hydrogens at 1st and 3nd positions, they are indicated by 1,3-dihydro and the base name is
1,3-dihydro-1,4-benzodiazepin-2-one
Similarly, it has side chains as
- 5-phenyl
- 7-nitro
Combining all prefixes and base name, the IUPAC name of nitrazepam is
7-nitro-5-phenyl-1,3-dihydro-1,4-benzodiazepin-2-one
Clonazepam
Clonazepam is another structure with nitro group at 7th position. It is similar with nitrazepam except an extra chloro group on phenyl ring.
It has phenyl ring at 5th position which is substituted with chloro group at 2nd position, hence name of the side chain is
5-(2-chlorophenyl)
Therefore all the side chains can be listed as
- 5-(2-chlorophenyl)
- 7-nitro
Hence the IUPAC name of clonazepam is
5-(2-chlorophenyl)-7-nitro-1,3-dihydro-1,4-benzodiazepin-2-one
Compared with nitrazepam, a change in alphabetical order can be observed. Here chlorophenyl group starts with letter "c" hence comes alphabetically first than nitro group as it starts with letter "n".
Chlordiazepoxide
Chlordiazepoxide is the first drug that was developed. Its structure little differ with other conventional benzodiazepines at 2nd position.
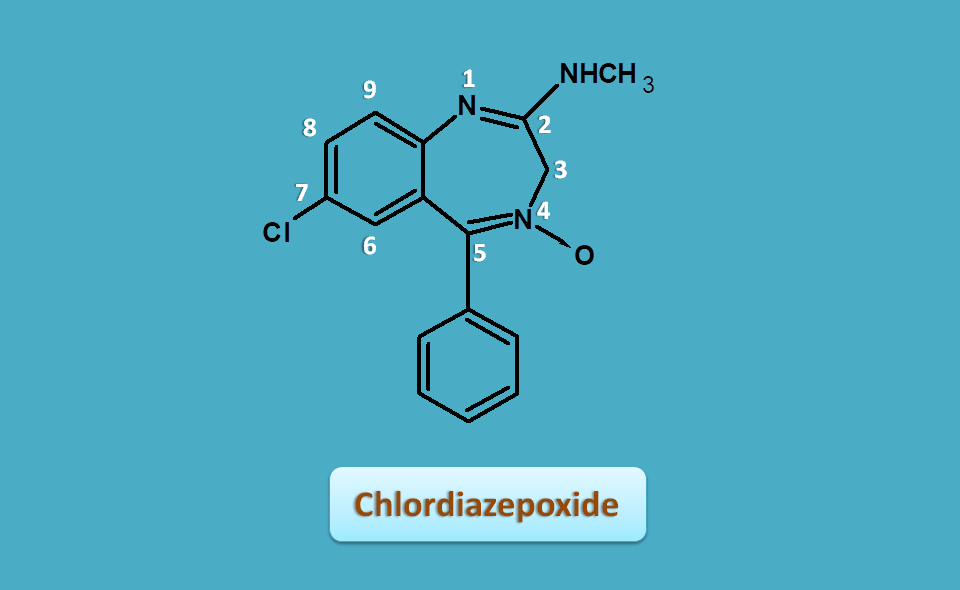
At 2nd position keto group is required for activity but this drug contains methyl amino group. Luckily this group can be replaced with keto group in-vivo preserving its activity. It also has N-oxide at 4th position.
Hence the side chains are
- 2-methylamino
- 5-phenyl
- 7-chloro
Hence the IUPAC name of chlordiazepoxide is
7-chloro-2-methylamino-5-phenyl-3H-1,4-benzodiazepin-4-oxide
Fused benzodiazepines
These drugs have an additional ring fused at 1st and 2nd position of the benzodiazepine ring. They can be of two types based on their fusion with triazole or imidazole.
- Triazolobenzodiazepines
- Alprazolam
- Triazolam
- Imidazolobenzodiazepines
- Midazolam
Alprazolam
Alprazolam is a fused ring system obtained from triazole and benzodiazepie rings.
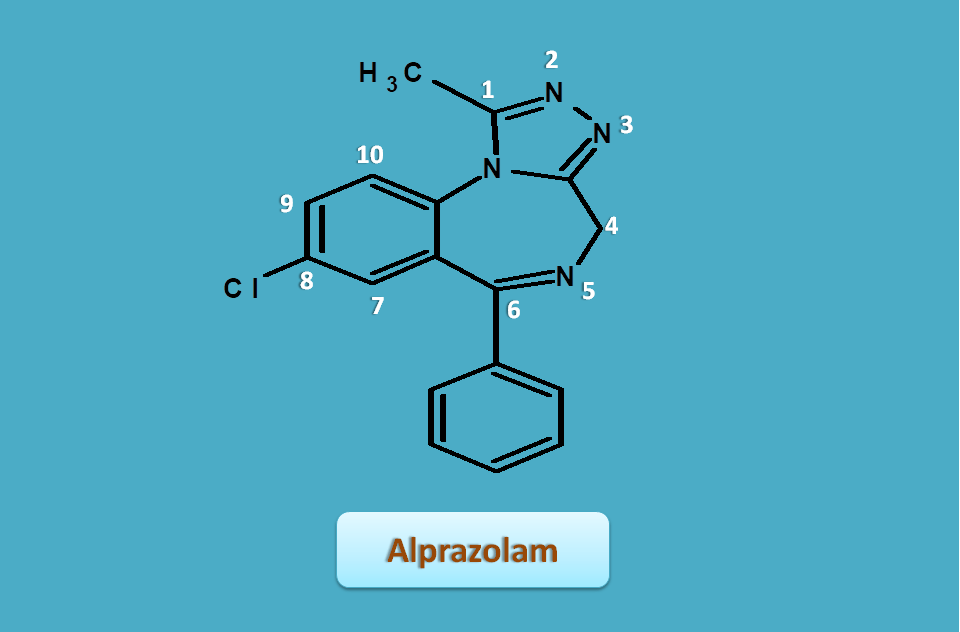
So let's see how they are fused and how to give numbering. First let's give numbering to two individual rings.
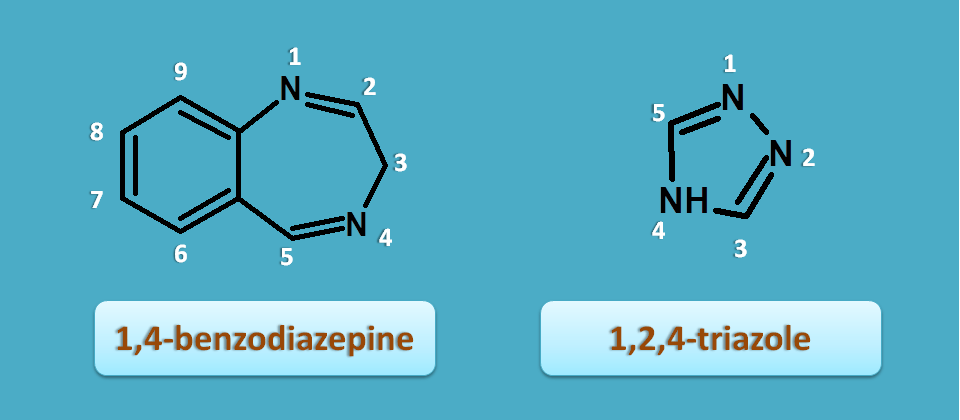
According to IUPAC, naming of the fused rings should be given by following rules
The ring fused on another ring is indicated by suffix "o"
Here it is indicated by using "triazolo".
The location of fusion is indicated by using location of fusion atoms in one ring and bond involved in fusion in another ring indicated by letter like a,b,c etc.
In another words, in one ring atoms should be labelled and in another bonds should be labelled.
As alprazolam is a triazolo benzodiazepine, let's give numbering to triazole ring and labelling to bonds in benzodiazepine.
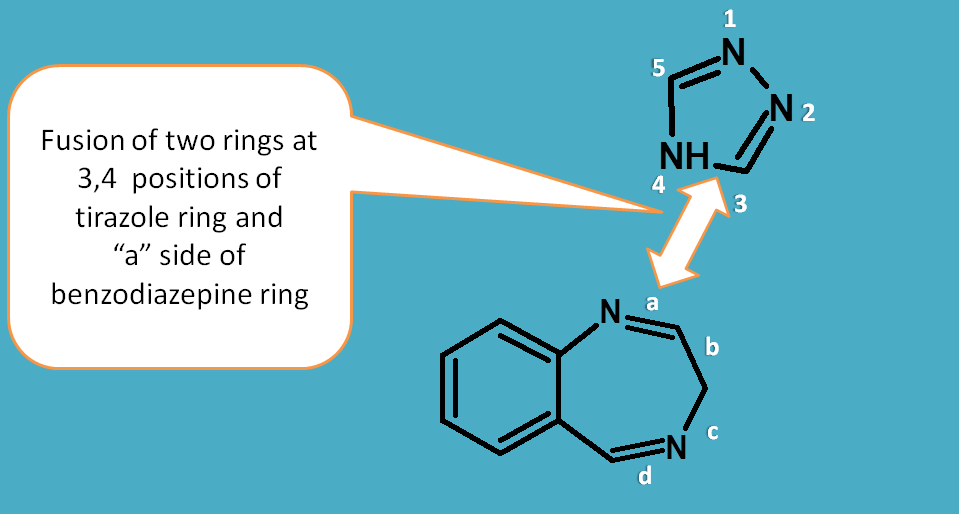
In alprazolam, the 3rd and 4th atoms of 1,2-4-triazole ring are fused with "a" bond on benzodiazepine. Therefore name of the fused ring system can be written as
[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
The location of fusion atoms in one ring should be indicated in decreasing order as 4,3 but not as 3,4.
Numbering
Now, let's see the numbering of complete structure alprazolam
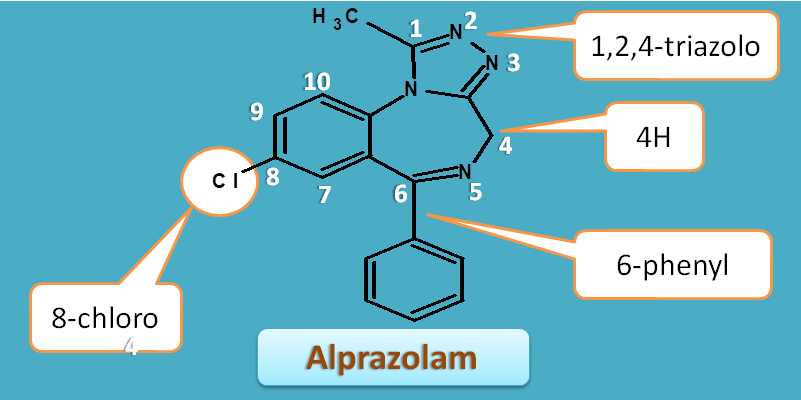
Same rules are applied as we discussed above, so numbering should be started from adjacent atom to the fusion atom. Again least number should be given to the hetero atoms with least sum of locants.
Therefore numbering starts at triazole ring. The 4th carbon has extra hydrogen and indicated by "4H".
Similarly side chains can be listed as
- 1-methyl
- 6-phenyl
- 8-chloro
Combining all, the IUPAC name of the alprazolam is
8-chloro-1-methyl-6-phenyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
You can notice that the location of side chains on the benzodiazepine ring were shifted by one number as compared with normal benzodiazepines.
- 3H --------- to ---> 4H
- 5-phenyl ---- to ---> 6-phenyl
- 7-chloro ---- to ---> 8-chloro
So numbering is given from triazole ring and bridge atoms are not numbered.
Triazolam
Triazolam is just similar to alprazolam with an extra chlorine on phenyl ring.
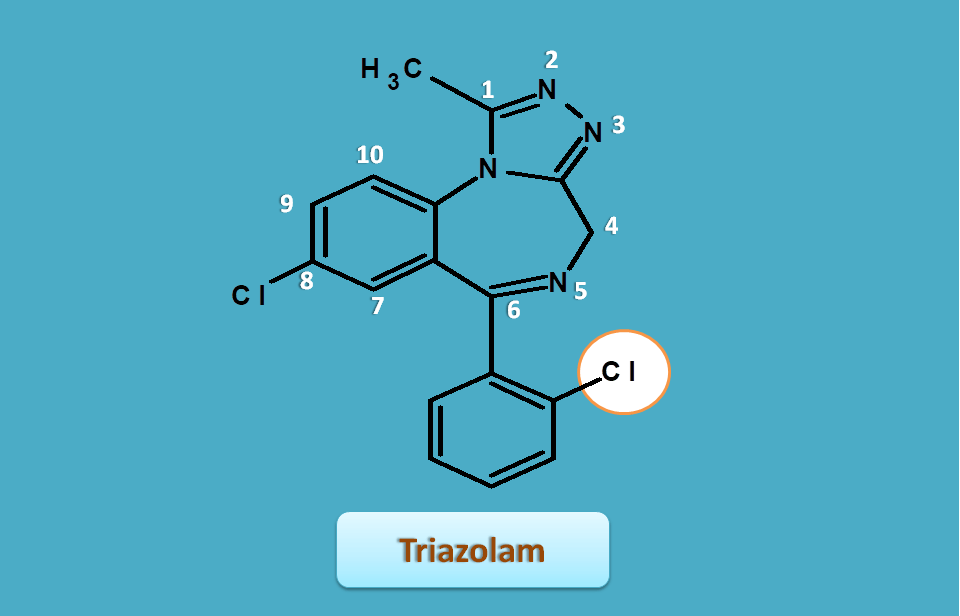
Hence base name is
[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
Similarly, side chains are
- 1-methyl
- 6-(2-chlorophenyl)
- 8-chloro
Therefore the IUPAC name of triazolam is
8-chloro-6-(2-chlorophenyl)-1-methyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
Midazolam
Midazolam has imidazole ring instead of triazole ring and a fluorine group on phenyl ring.
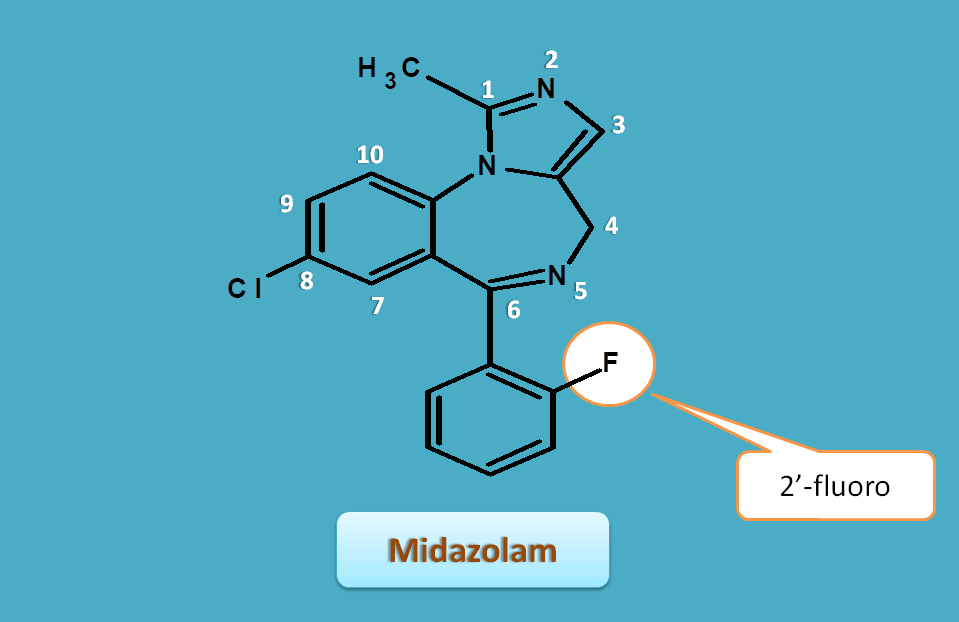
Here again the imidazole is given numbering and sides of benzodiazepine are labelled with letters a,b,c.
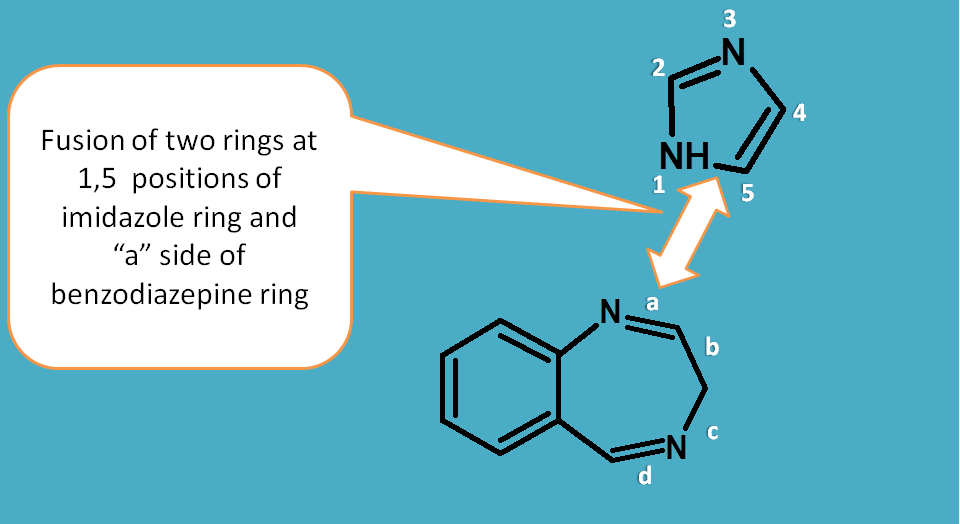
It can be noticed that 1st and 5th atoms of imidazole are fused with "a" side of benzodiazepine. Hence name of the fused ring is
imidazolo[1,5-a][1,4]benzodiazepine
Again it has extra hydroge at 4ht position hence "4H".
6th position it has phenyl group with fluoro group at ortho position, hence 6-(2-fluorophenyl).
Side chains can be indicated as
- 1-methyl
- 6-(2-fluorophenyl)
- 8-chloro
So complete name of the midazolam is
8-chloro-6-(2-fluorophenyl)-1-methyl-4H-imidazolo[1,5-a][1,4]benzodiazepine
Midazolam is not used as anxiolytic instead used as IV anaesthetic agent.
That's about the nomenclature of benzodiazepines. Share this article if you like this post.