Lewis dot structure – How to write?
by egpat 21 Mar 2019
Do you think anytime how the atoms form bonds to bring molecules? Particularly in organic chemistry, most of the times a bond is formed by sharing of electrons between the atoms. Which electrons they share and how it happens? For all these questions the best answer can be obtained by visualising every atom in the molecule with Lewis dot structures. Here we will go initially with drawing of lewis structure of atoms and then with molecules.
Octet rule
Whether atoms are stable in nature?
Definitely, they are not stable. Many of the atoms do not have complete filling of orbitals in their outer orbit and therefore not stable and highly reactive.
What’s that complete filling? Is there any fixed number assigned to each orbit?
Yes, there it is. Let’s go in detail.
According to octet rule, an atom will achieve stable configuration if it is filled with 8 electrons in outer shell.
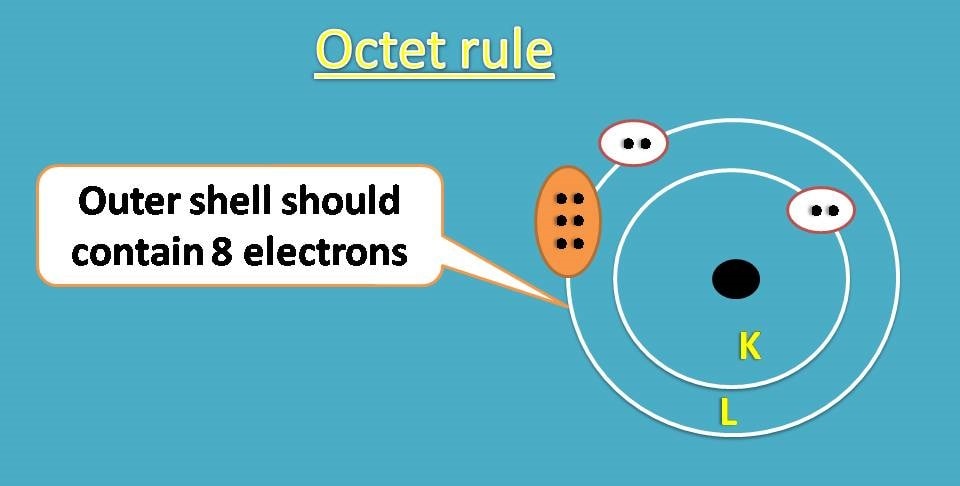
Is there any such atom exists in nature?
Why not? We have such atoms in the periodic table that acquire stable configuration commonly called as noble gases.
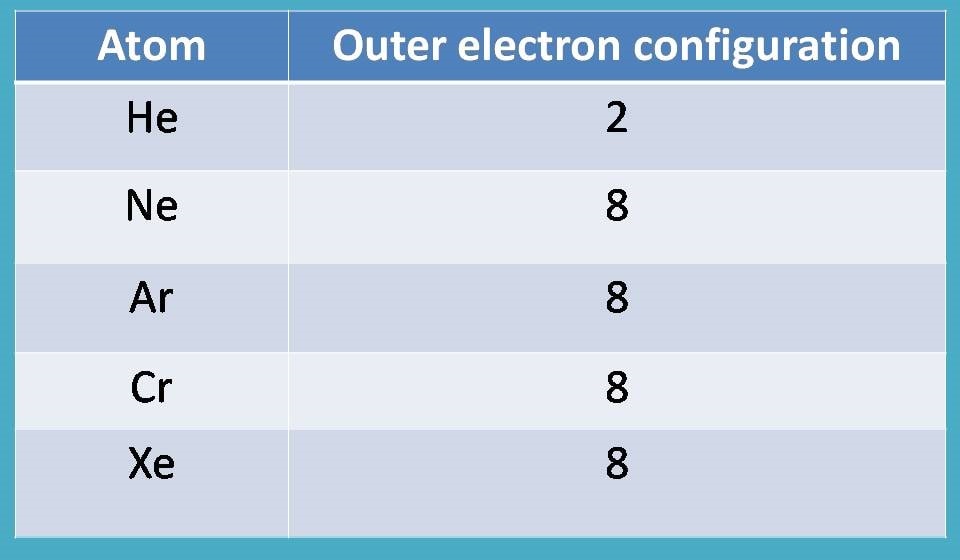
What about other atoms?
Since most of the atoms are not completely filled with electrons, they are deficient in completing octet but still they try to get it by interacting with other atoms.
This can be achieved in two ways. First, one of the atoms can donate and another atom can accept electrons each acquiring stable octet configuration.
For example, sodium with atomic number being 11 has electronic configuration as 1s2 2s2 2p6 and 3s1.
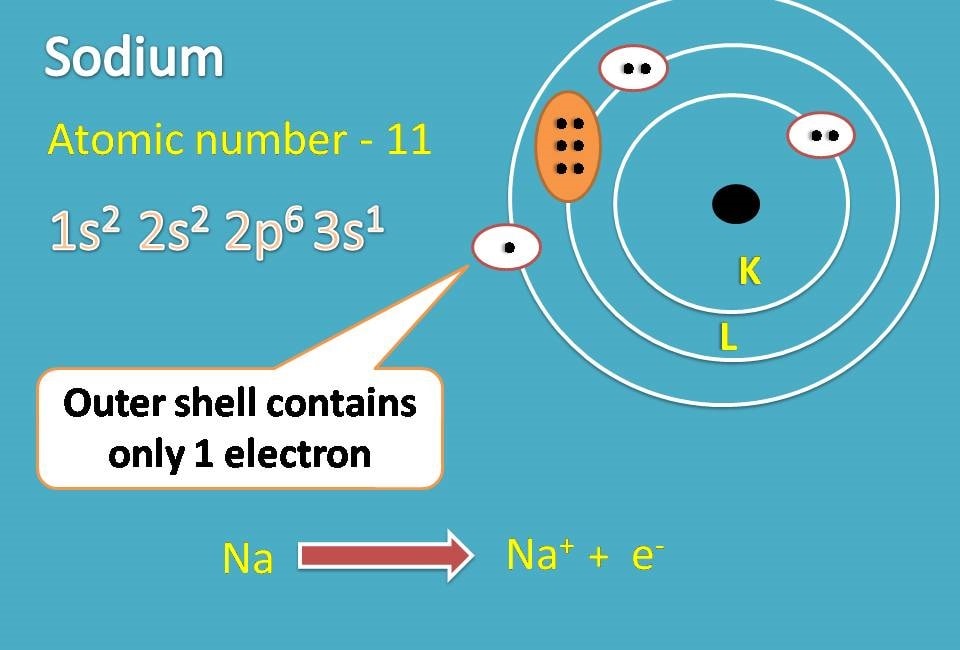
If you observe outer shell contains only one electron while 2nd shell contains 8 electrons. So if it donates one electron, second shell becomes outer shell and it achieves noble gas configuration.
In this way sodium becomes sodium ion.
Similarly, chlorine with atomic number 17 has electronic configuration as 1s2 2s2 2p6 3s2 3p5. Here the outer shell contains 7 electrons and it can easily accept one electron to complete octet.
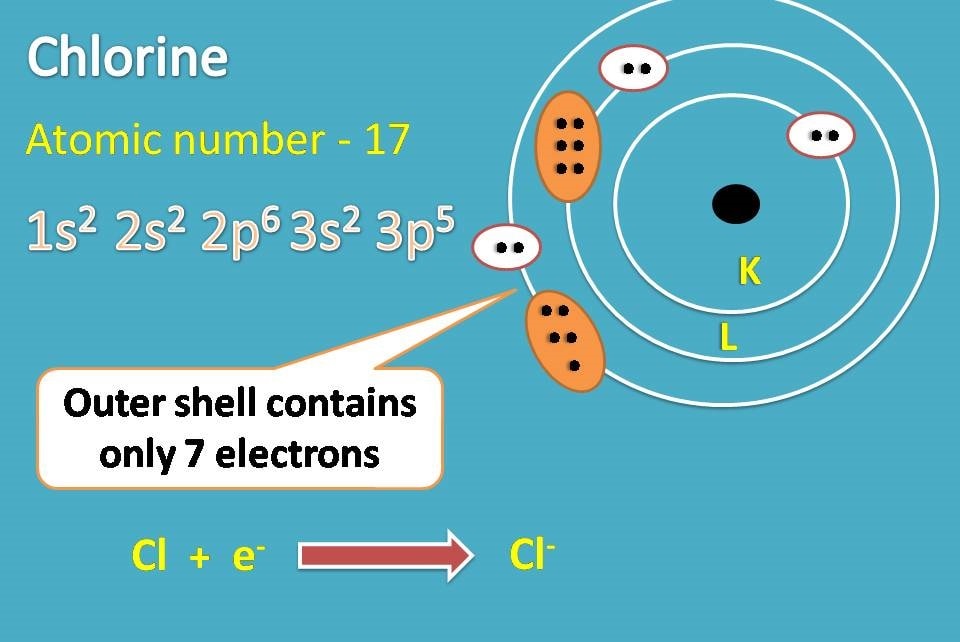
Thus chlorine forms chloride ion. Now the sodium ion and chloride ion can form an ionic bond due to electrostatic attraction.
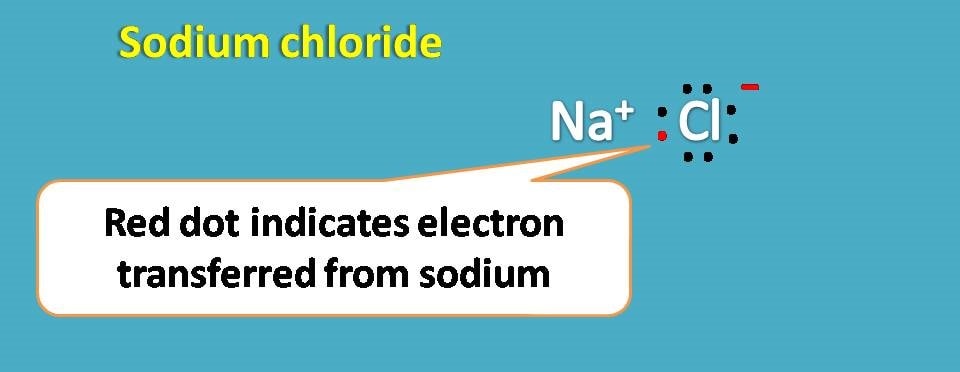
Second way of getting noble gas configuration is by sharing of electrons.
Surprisingly, atoms are so friendly that they share electrons with each other making complete octet in their outer orbitals.
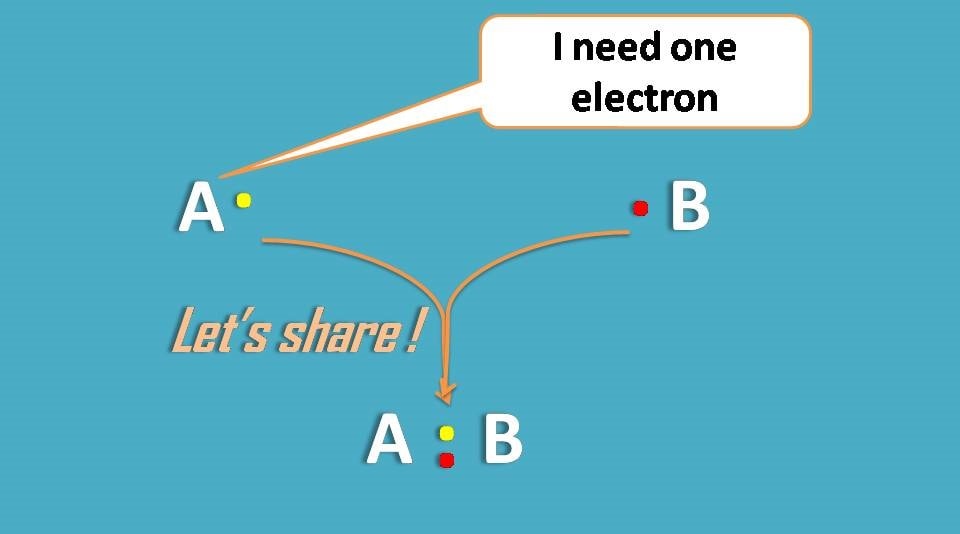
Lewis dot structures are so helpful in understating the concept well, so we will proceed our further discussion with them.
Lewis dot structure of noble gases
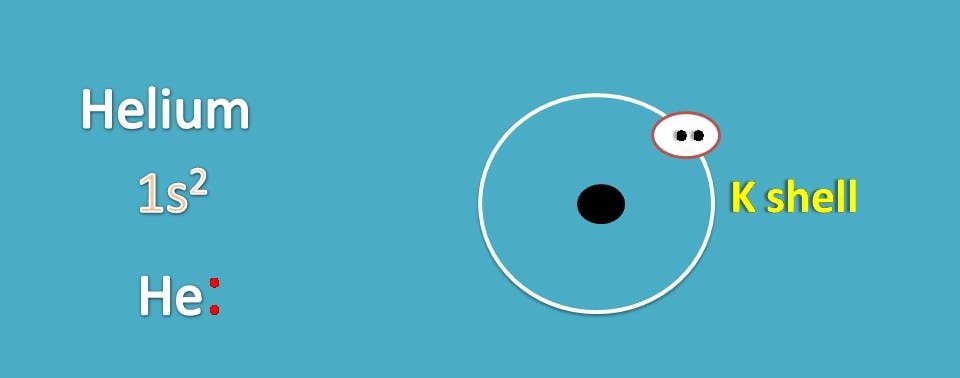
First we will see how to draw Lewis dot structures for noble gases. Let’s start with helium. Its atomic number is 2 and electronic configuration is 1s2.
It contains 2 electrons in K shell.
Next, neon. It has atomic number ten and two shells such as k and L. Each shell is loaded with maximum number of electrons such as 2 and 8 respectively.
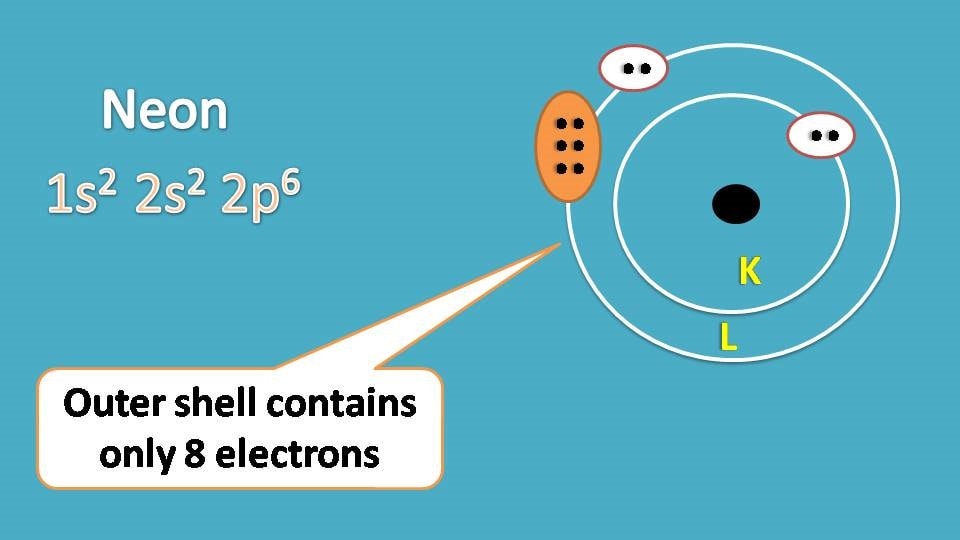
Lewis dot structure of neon can be written as follows.

Surprisingly we have shown only 8 electrons in the lewis structure of neon.
Why we have omitted the other two electrons, are they not important?
Definitely they are important and without them neon won’t exists. But for practical purpose we can’t show all the electrons in an atom in the lewis structure.
Let’s dig a bit more into it.
Neon has two shells where K shell is the inner shell while L shell is the outer shell. Generally electrons in the outer shell are more reactive and therefore the stability of the element depends on the filling of electrons in outer shell. So it is sufficient to represent the outer electrons in the lewis structure.
Lewis dot structure will be similar for all noble gases showing 8 electrons in outer orbitals.

Lewis dot structure of molecules
Now we will go with molecules and we will see how we can represent them with lewis dot structures.
Let’s start with simple atom, hydrogen. It has atomic number as 1 and therefore its electronic configuaration is 1s1.
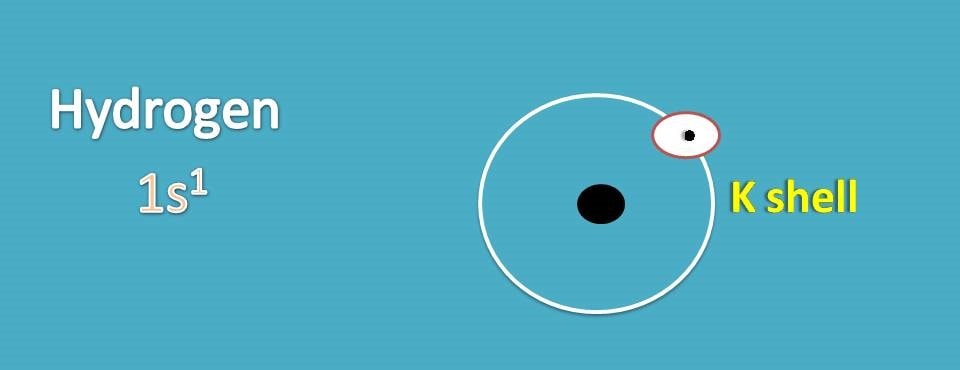
It has electrons filled only in K shell which is the outer orbital for the hydrogen. Now we can represent lewis dot structure for hydrogen by indicating each electron with a dot.
Since K shell should have two electrons for stable configuration, hydrogen has a deficiency of electrons in the outer orbit. Therefore it can’t exist as it is but it can combine with another hydrogen to form a stable electronic configuration.
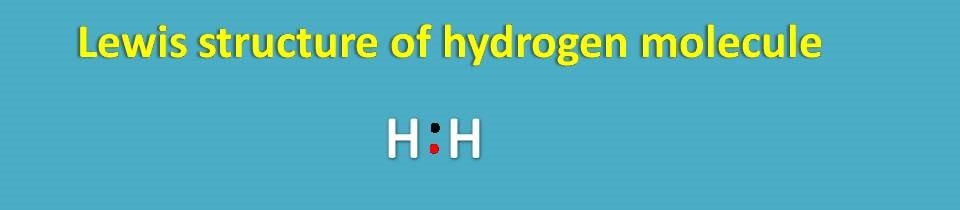
You can see here in the lewis dot structure, each hydrogen has one electron shown by different color. In this way, each hydrogen has attained stable configuration by sharing electrons with each other.
Hydrogen can also share its electrons with other atoms which are deficient with one electron from stable noble gas configuration.

Here hydrogen is sharing its electrons with chlorine and each atom attained stable configuration. Hydrogen has totally two electrons while chlorine has 8 electrons in the outer shell.
note: here chlorine is not forming ionic bond rather it shares its electron with hydrogen to form covalent bond.
That’s simple is the lewis dot structure.
Here we go with few examples of molecules and their lewis structures.
Lewis structure for water
Water has the molecular formula H2O. The atomic number of oxygen is 8 with electronic configuration as 1s2 2s2 2p4. It has totally 6 electrons in the outer shell and therefore needs two more electrons to complete octet.
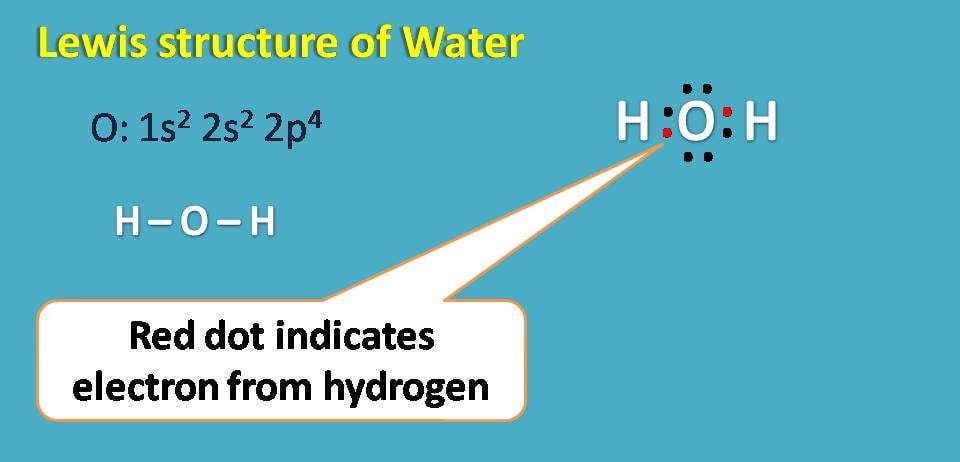
Therefore it can form a covalent bond with two hydrogen atoms forming water molecule.
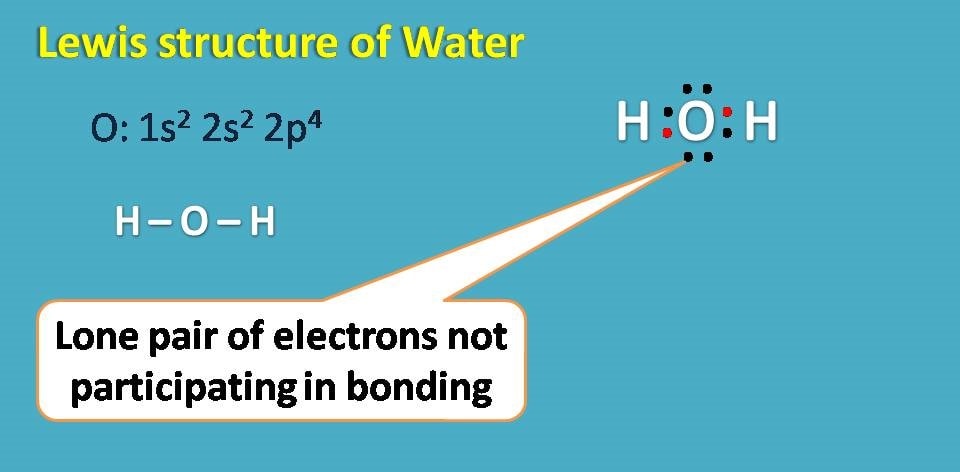
Interestingly, even oxygen has 6 electrons in outer shell all these electrons are not participating in covalent bonding with hydrogen. Those electrons which doesn’t involve in bonding are generally treated as non-bonding electrons or simply lone pair of electrons. In water molecule you can observe two lone pair of electrons.
Lewis structure for ammonia
Ammonia has molecular formula as NH3 with nitrogen as central atom. Atomic number of nitrogen is seven and electronic configuratoin is 1s2 2s2 2p3. Since it has 5 electrons in outer shell, it needs further three electrons hence it forms covalent bond with three hydrogen atoms.
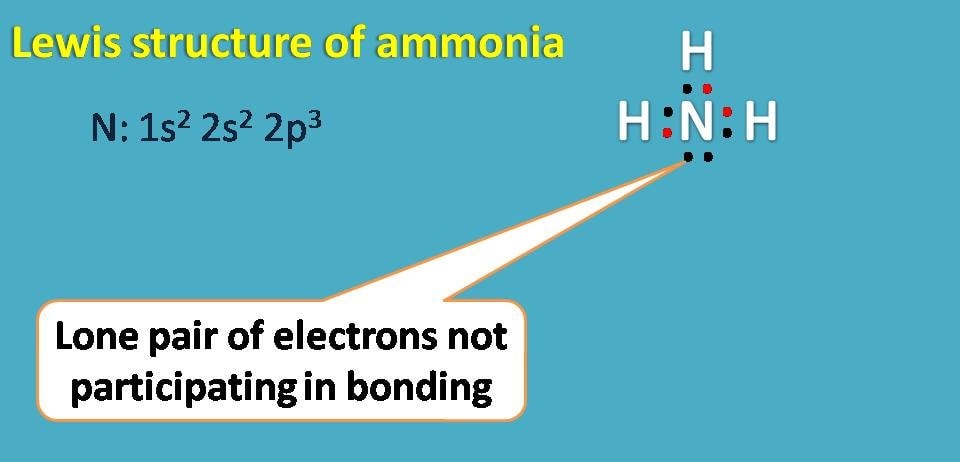
Here again you can find one non-bonding electron on nitrogen.
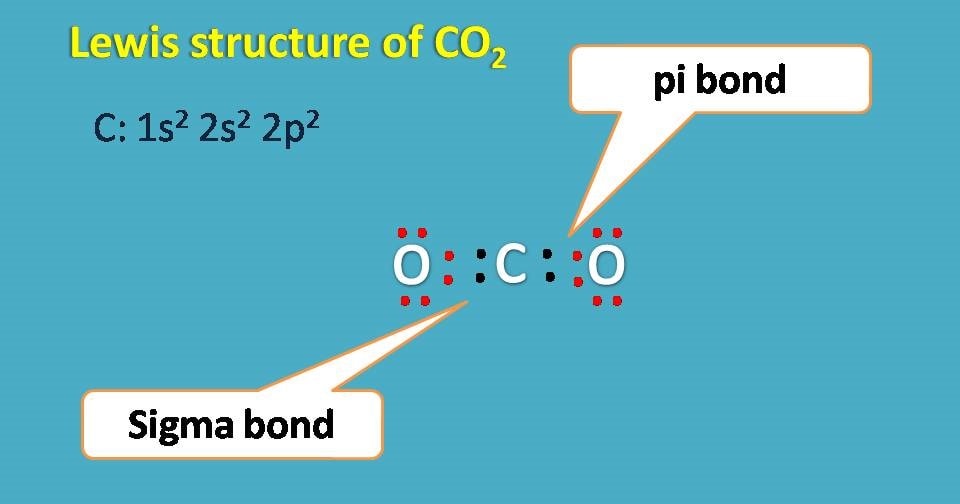
Lewis structure of carbon dioxide
Carbon dioxide has molecular formula as CO2 and here the central atom is carbon with electronic configuration 1s2 2s2 2p2. It has 4 electrons in the outer orbital and needs further 4 electrons to complete octet. Therefore each oxygen shares two electrons .
But, how its possible as each covalent bond is formed by sharing of only one electron from each atom?
The answer is simple. Even one electron is shared by sigma bond another electron is shared by pi bond between oxygen and carbon.
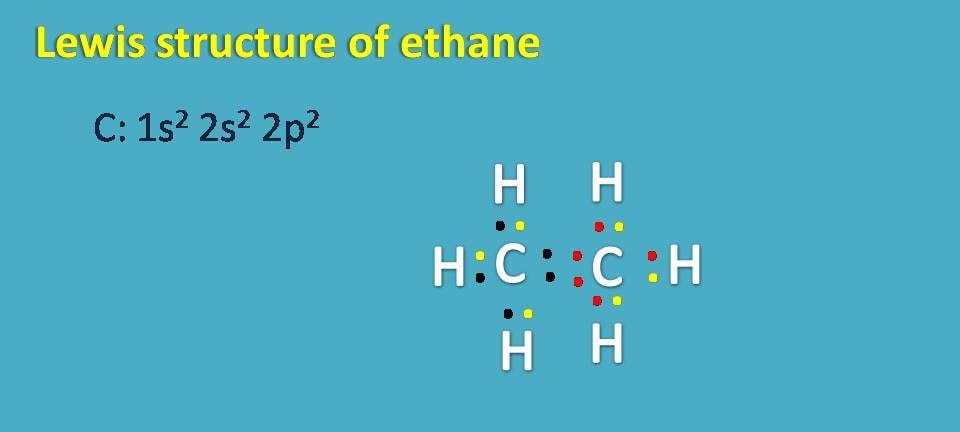
Lewis structure of ethane
Finally, lewis dot structure of ethane can also be written in similar way. Here each carbon shares its electrons with three hydrogens and with one carbon thereby gets 8 electons totally.
Conclusion
In this way, we can write lewis dot structures very easily by knowing the electronic configuration of the atom. Every atom gets stability once it achieves 8 electrons in its outer orbital. This can be achieved by complete transfer of electrons from one atom to other forming ionic bond or even by sharing electrons between atoms forming covalent bond. That's it. If you like the post, please share it with your friends.