Priority order of functional groups in IUPAC nomenclature
by egpat 08-08-2017
The first step in providing chemical name for compounds in organic chemistry is to identify the principle functional group for which learning priority order of functional groups in IUPAC nomenclature is a key aspect.
IUPAC nomenclature mainly uses substitutive nomenclature i.e. names of all aliphatic compounds are derived from the names of corresponding hydrocarbon by replacement of suffix “e” with corresponding suffix of functional group.
For example in the following structure hydrogen is replaced by hydroxyl group.
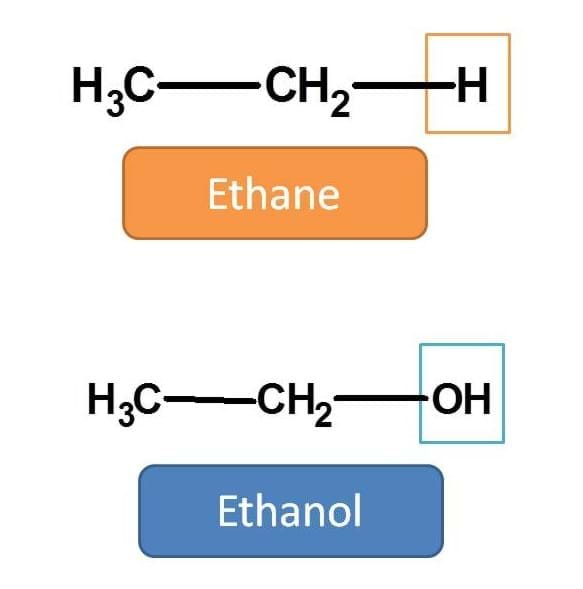
Hence we should replace the suffix “e” with “ol” i.e. ethane + ol=ethanol
A compound may have one or more function groups in which case one of the group is to be selected as principle functional group.
How it can be done?
Yes, we can compare the relative positions of groups in functional group priority table and pick that group which is in the top position considering it as principle functional group.
Hence determining the priority order is a key step in naming of the organic compounds.
Here we will see how to determine the priority order of functional groups in IUPAC nomenclature along with few examples.
Functional group priority list
We have lot of functional groups in organic chemistry such as acids, acid derivatives, aldehydes, ketones, alcohols, amines and so many other groups. A group which act as principle functional group in one structure may be treated as side chain in other instances.
<For example,
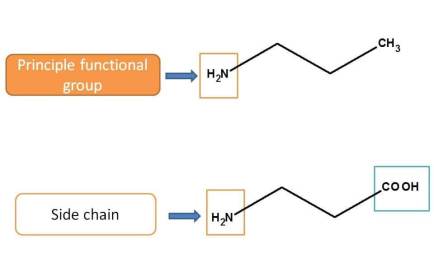
Here amine group is acting as principle functional group in first structure whereas it acts as side chain in the second structure as carboxylic acid given more priority over amine group.
The priority order of functional groups in IUPAC nomenclature is based on a relative scale where all functional groups are arranged in the decreasing order of preference.
When a group is considered as principle functional group, it is indicated by suffix and when it acts as side chain, it is indicated by prefix. So, most of the groups will have both prefix and suffix. But in few cases, a group may always be treated as side chain due to least priority.
That’s fine.
But is there any easy way to remember priority of functional groups in IUPAC nomenclature?
Yes, we have. We can simple apply a little logic to functional group priority table to remember them in easiest way without any confusion.
To make discussion more clear, let’s divide all functional groups in to three categories.
Functional groups with different priority:
- Here all functional groups are arranged in decreasing priority order
- They contain both prefix and suffix
Functional groups with equal priority :
- Here all functional groups have equal priority
- They have suffix only
Functional groups with no priority :
- Here functional groups have no priority and always considered as side chains
- They have prefix only
By classifying in this way, you can easily identify in which table a functional group falls and you can remember the entire priority order of functional groups in IUPAC nomenclature. Let's start our discussion with each category.
Functional groups with equal priority
For simplification, we have included widely used functional groups here.
| Functional group | Formula | Prefix | Suffix |
|---|---|---|---|
| Carboxylic acid | COOH | Carboxy | -oic acid |
| Sulfonic acid | SO3H | Sulfo | sulfonic acid |
| Sulfinic acid | SO2H | Sulfino | Sulfinic acid |
| Sulfenic acid | SOH | Sulfeno | Sulfenic acid |
| Anhydrides | -COOCO- | - | -oic anhydride |
| Ester | COOR | Alkoxy carbonyl | -oate |
| Acid halide | COX | Haloformyl | -oyl halide |
| Amide | CONH2 | Carbamoyl | -amide |
| Hydrazides | -CO-NH-NH2 | -carbonyl-hydrazino- | -hydrazide |
| Imides | -CO-NH-CO- | R-imido | -carboximide |
| Imidines | -C(=NH)-NH2 | Amidino | -amidine |
| Nitrile | CN | Cyano | -nitrile |
| Aldehyde | CHO | Formyl (oxo) | -al |
| Ketone | CO | Oxo- | -one |
| Alcohol | OH | Hydroxy- | -ol |
| Thiols | -SH | Mercapto- | -thiol |
| Amine | NH2 | Amino- | -amine |
| Imines | -NH- | Imino- | -imine |
| Hydrazines | -NH-NH2 | Hydrazino- | -hydrazine |
| Ether | -OR | Alkoxy- | ether |
Let's remember this category in easy way.
The above statement is only intended for easy remembering and easy analysing of functional group priority table. Let's assume a double bond as two attachments or two single bonds. We can divide the functional groups in three types based on the number of linkages with heteroatom.
- Functional groups having three bonds with heteroatom
- Functional groups having two bonds with heteroatom
- Functional groups having single bond with heteroatom
So, let's start with functional groups attached with more number of bonds with heteroatom.
First, acid and acid derivatives have totally three bonds with heteroatom (-O). Among these, acids are given more preference than their derivatives. Hence the order is
acids > acid derivatives
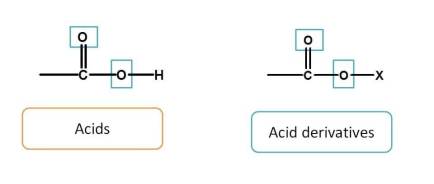
Since sulfur is congener of carbon, we can also add sulfur derived acids just after the carboxylic acids. Hence order is
carboxylic acids > sulfonic acids > acid derivatives > sulfonic acid derivatives
Next, nitriles have three bonds with heteroatom (-N). Observe here nitriles have nitrogen as heteroatom and less preferred than acids which have oxygen as heteroatom. You can remember it as "more the electronegativity more the preference". Oxygen is more electronegative than nitrogen and more preferred.
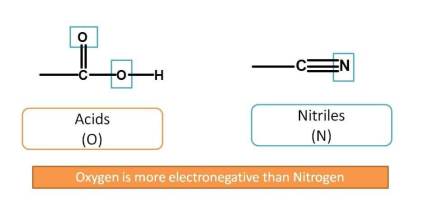
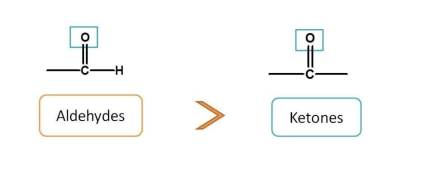
Now, next functional groups with two bonds with heteroatom are aldehydes and ketones. Aldehyde is given more preference over ketone. Now the order is
carboxylic acids > sulfonic acids > acid derivatives > sulfonic acid derivatives > Nitriles > Aldehydes > Ketones
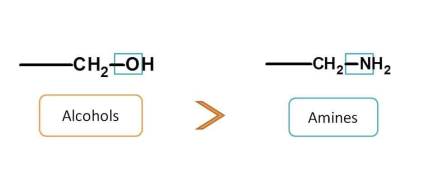
Finally groups having single bond with heteroatom include alcohols (-O) and amines (-N). Alcohols have more elctronegative atom (O) than amines (N) and more preferred. So, final order is
carboxylic acids > sulfonic acids > acid derivatives > sulfonic acid derivatives > Nitriles > Aldehydes > Ketones > Alcohols > Amines
The above discussion is an oversimplification to easily remember the order. Now let's go in detail about each group along with examples.
Functional groups having three bonds with hetero atom
So, functional groups connected by 3 bonds to heteroatom are acids and acid derivatives. Let's start with acids.
Carboxylic acids
These are the first functional groups that are given highest preference. Of course, quaternary ammonium compounds are still given more preference than acids, but to simplify they are not included here.
Does carboxylic acid require any prefix ?
As we are discussing that carboxylic acids are top priority groups hence always treated as principle functional group indicated by suffix, then what is the need of prefix? Is it required? The answer is yes, they require. Even carboxylic acids are top priority groups in few situations they may act as side chains. Let's take this example.
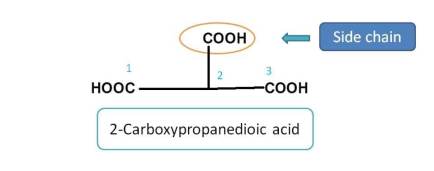
Here, the principle functional groups is carboxylic acid and the parent chain is three carbon chain including two carboxylic acids. So one of the carboxylic acid group is treated as side chain. Hence it should be indicated by prefix 2-carboxy. Therefore the name of the compound is 2-Carboxypropanedioic acid.
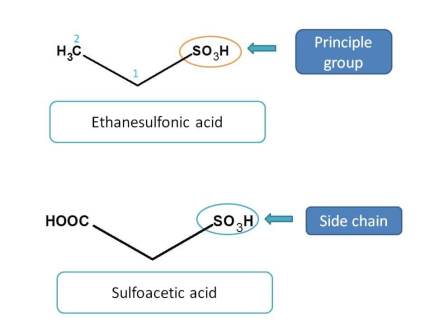
Sulfonic acids and their analogues
Next preference is given to sulfonic acids. But sulfur, not like carbon, can exhibit variable oxidation states and can exist as sulfonic acid, sulfinic acid and sulfenic acid.
Acid derivatives
Acid derivatives are given next preference with the following order.
Anhydrides > esters > acid halides > amides
Anhydrides does not have prefix as the oxygen shows valency 2 not 4 as carbon. So it cannot be attached further and doesn't act as side chain.
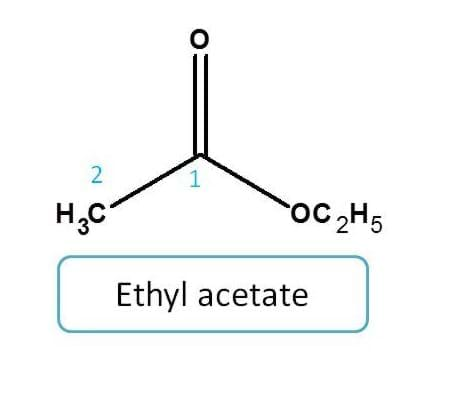
Example for ester
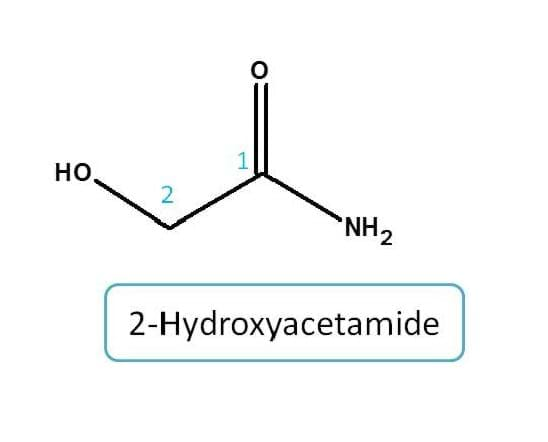
Example for amide
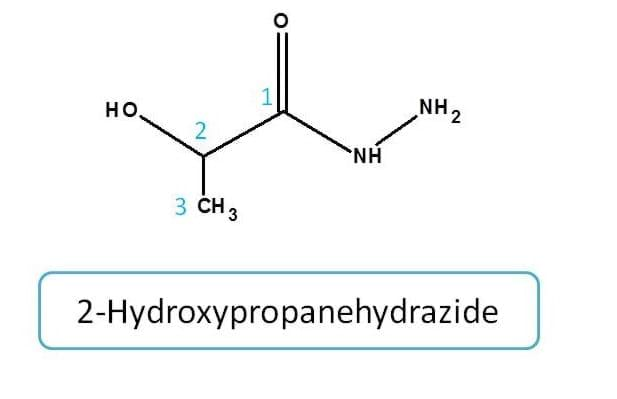
Amide analogues
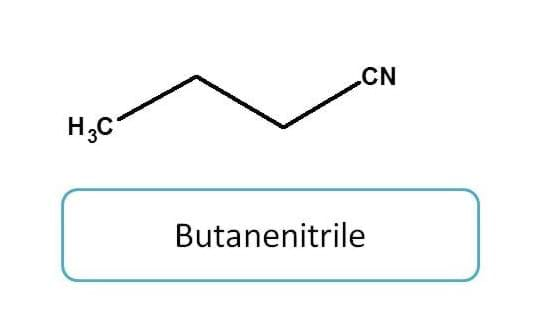
Nitriles
Functional groups having two bonds with hetero atom
Now let's go to functional groups attached with two bonds which include aldehydes and ketones.
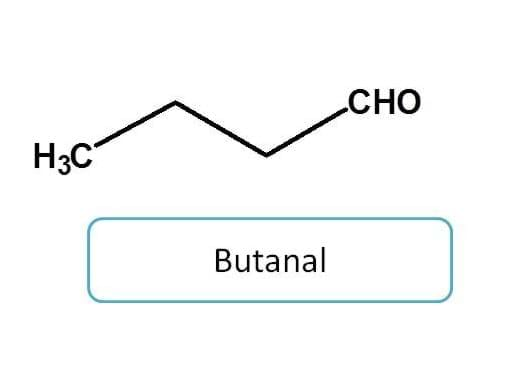
Aldehyde when used as side chain, can indicated any of the two prefixes according to situation.
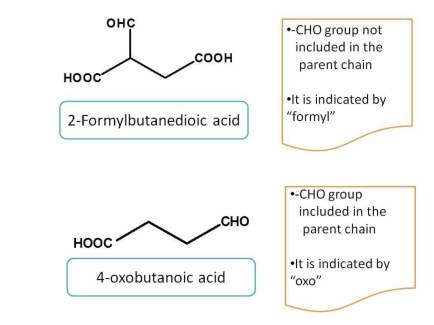
If carbon is counted, it is indicated by the prefix "oxo-" otherwise as "formyl-"
Functional groups having single bond with hetero atom
Finally, functional groups attached with single bond include alcohols, amines and their derivatives.
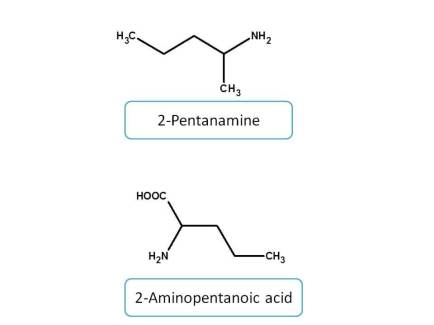
Among the amine derived functional groups, the priority is given as follows.
Amines > Imines > hydrazines
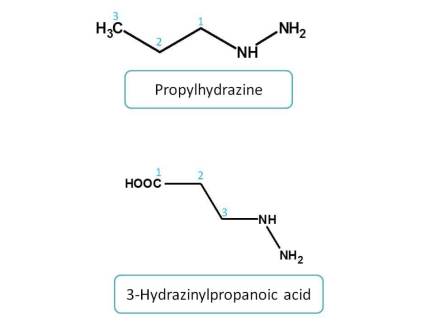
Functional groups with equal priority :
| Functional group | Formula | Prefix | Suffix |
|---|---|---|---|
| Alkene | >C=C< | - | -ene |
| Alkyne | -C≡C- | - | -yne |
Here we can find only two groups which are always treated as suffix. They are alkenes and alkynes. Two important points are to be noted here. First, both the groups have equal priority, So which act as the principle functional group is decided by other rules. Second, the suffix is used by replacing the "-ane" by either "-ene" or "-yne". (In the first category, we have replaced only last character i.e., "-e" with suffix like "-oic acid")
Order of priority
As we have discussed earlier, the order of priority depends on the situation. Let's take one example.
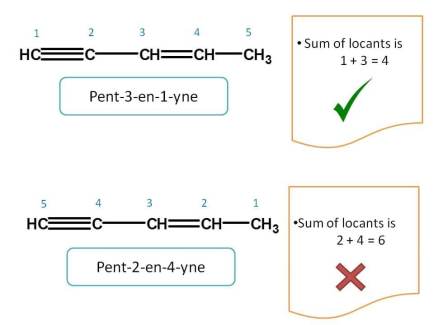
First we have to check for lowest sum of locants. In the above example, when numbering is given from right to left, the sum of locants of triple bond and double bond is 1 + 3=4 which is lower than the sum 2 + 4=6 obtained from other direction. Hence first direction is correct and name of the compound is Pent-3-en-1-yne.
Note here that even least locant is given to "yne", still the suffixes are arranged in alphabetical order.
Now let's take another example.
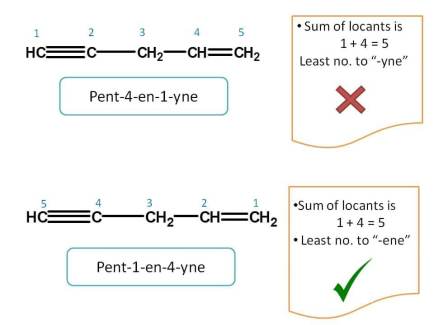
Just like above example, let's apply lowest sum rule. The sum of the locants is 1 + 4=5 from both the directions. So we have to apply next rule.
The group name which comes alphabetically first should be given more preference.
So according to above rule, "-ene" comes alphabetically first than "-yne" and hence should be given first preference. Hence name of the compound is Pent-1-en-4-yne
Functional groups with no priority
| Functional group | Prefix |
|---|---|
| Cl | Chloro |
| Br | Bromo |
| I | Iodo |
| F | Fluoro |
| R | Alkyl |
| OR | Alkoxy |
| -N2- | Diazo- |
| -N3 | Azido- |
| -NO | Nitroso |
| -NO2 | Nitro |
This category contains all the groups which mainly exist as side chains and they doesn't have any priority, so numbering is governed by lowest sum rule.
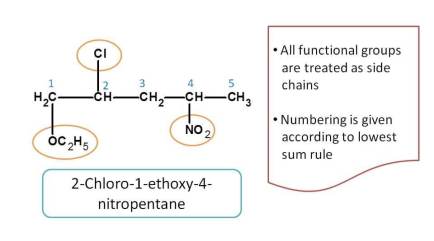
Here all the functional groups such as nitro, alkoxy and chloro groups have no priority and always considered as side chains. So the numbering is given based on the lowest sum of locants and prefixes are arranged in alphabetical order.
Here is a video on how to remember the priority order in easy way.
So to name an organic compound you should know the exact position of group in the function group priority table. But without applying logic remembering this list is a daunting task.
By proper reasoning and classifying the groups into three categories you can easily remember the priority order of functional groups in IUPAC nomenclature.