Sequence rules for ranking groups
by egpat 28 Mar 2019
Sequence rules will give you the power to label the orientation of the groups in the space around a stereocenter. For instance, a chiral carbon will have four different groups attached to it each oriented in a specific way to produce a pair of enantiomers. This spatial arrangement of the groups in the space can be studied by ranking these groups with sequence rules.
Is that part of IUPAC nomenclature?
No, it’s completely different from that. Sequence rules are designed by three scientists hence this nomenclature is commonly termed as Cahn-Ingold –Prelog system. These rules are used to provide ranking to the four groups attached to the chiral carbon.
In IUPAC we arrange the functional groups in the preferential order by using IUPAC priority order but here we follow sequence rules to rank them. Once the ranking is given we can find the suitable arrangement and label them as R and S enantiomers
First of all let’s go with each sequence rule.
Sequence rule 1
First sequence rule states that
It’s a simple rule and according to this we should give the ranking based on the atomic number of the element attached to chiral carbon. Let’s take on example.
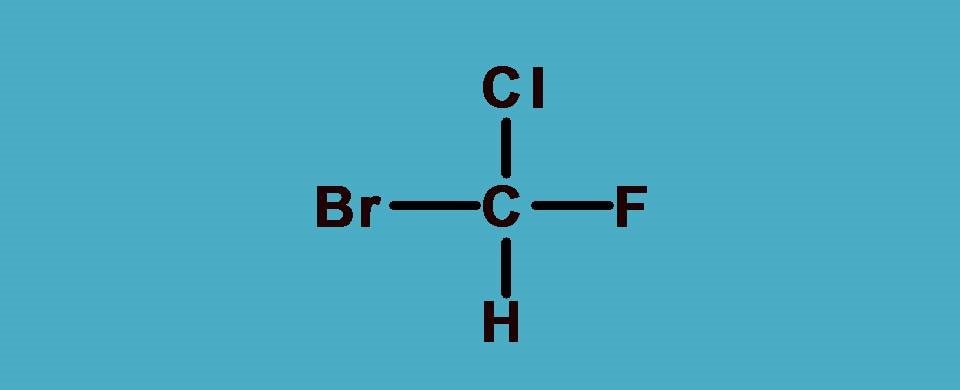
What are the groups attached to chiral carbon? Here the groups are -F,-Cl, -Br and hydrogen. As you know that -F,-Cl, -Br all fall in a group in the periodic table and atomic number increases as we go down in a group. So arranging them in the order of atomic numbers,
-Br > -Cl > -F > -H
Hydrogen is always given least preference as it the atom with least atomic number. Now you can see that all the elements attached to the chiral carbon are given ranking according to sequence rule 1.
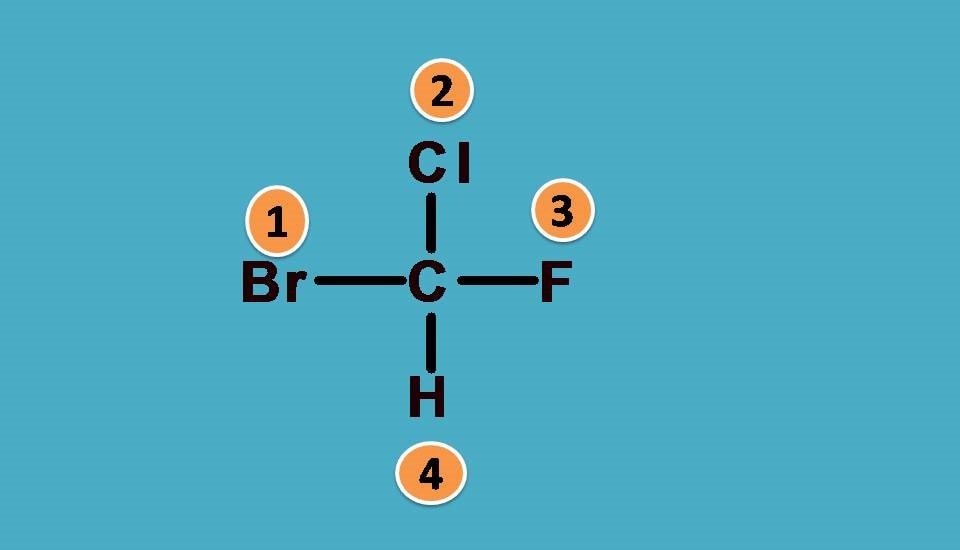
Let’s take another example.
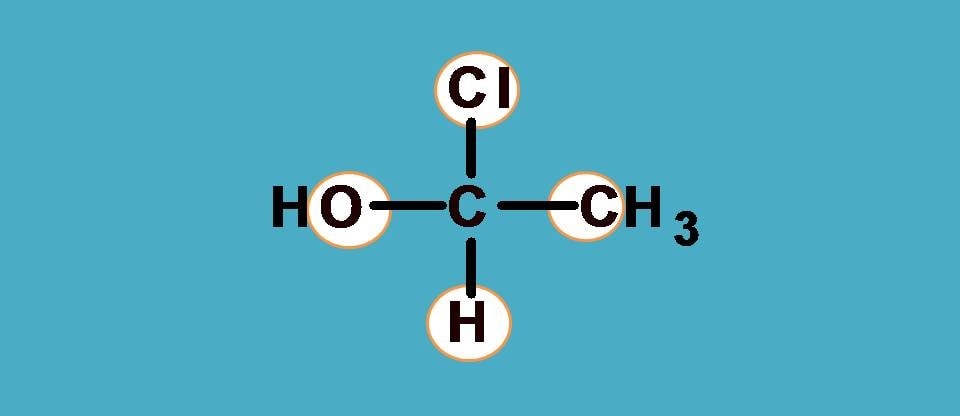
This time you can see groups rather than simple atoms attached to chiral carbon. Then how to proceed?
Here we have to consider the atom that is directly attached to chiral carbon.
Going with that logic, the first elements attached to chiral carbon in the above structure are -C from -CH3 and -O from -OH. Other two are single elements being Cl and H.
What next? Arrange them in order of decreasing atomic number. Who comes first? Definitely it is -Cl followed by -O and -C. So the ranking order is -Cl > -OH > -CH3 > -H.
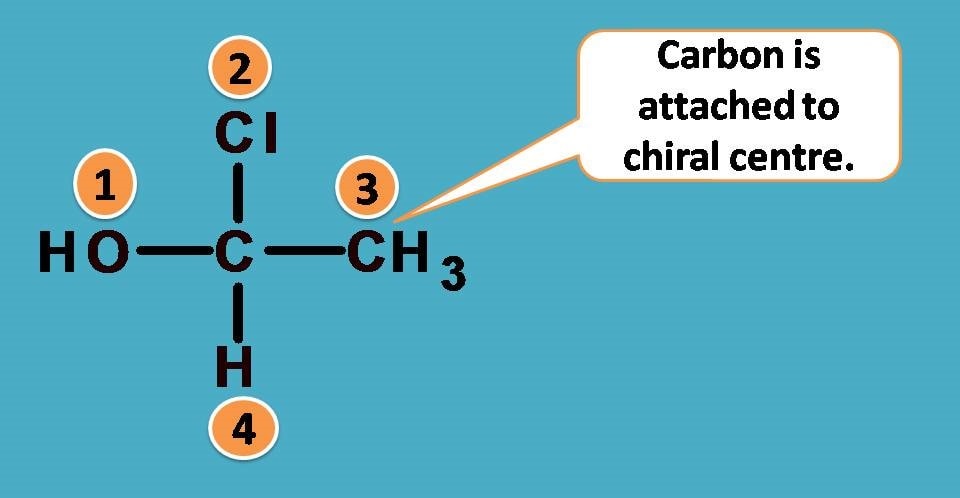
That’s fine. Another example here follows.
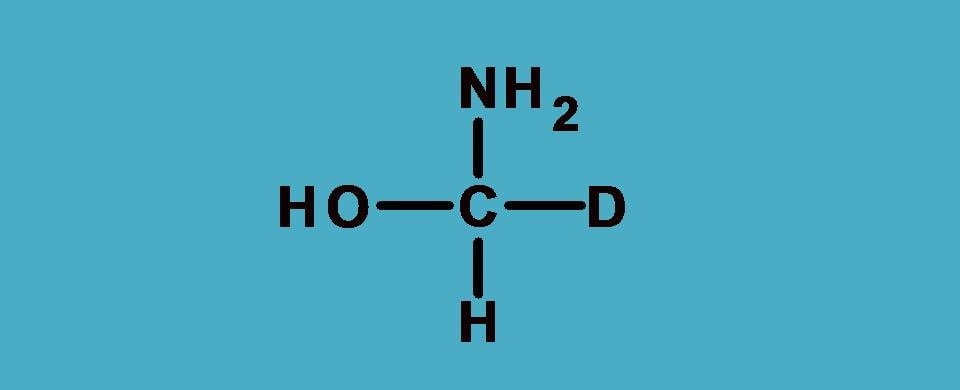
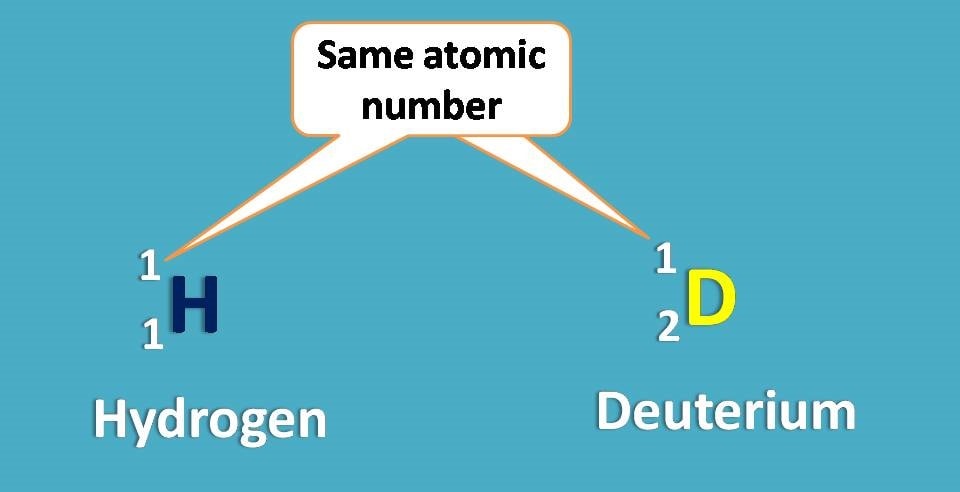
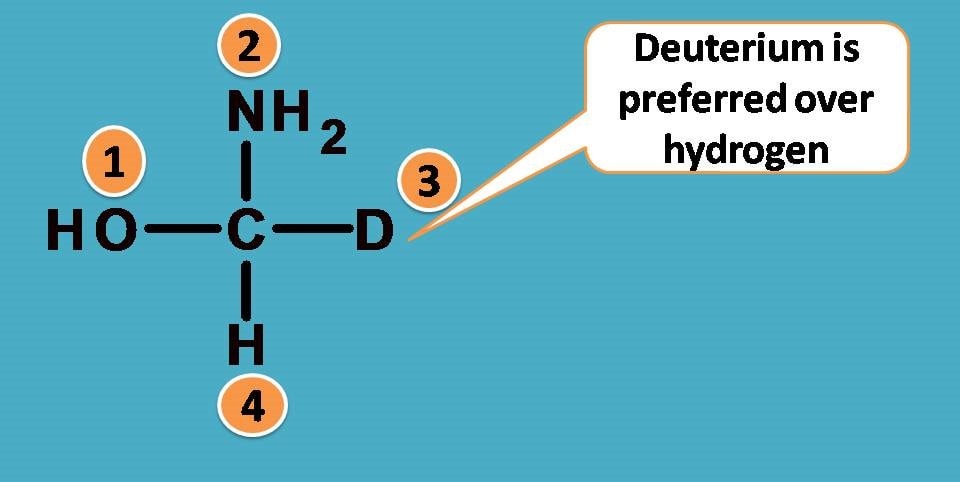
Again -O and -N are the first elements attached to central carbon from two groups –OH and –NH2. What about other two? One is hydrogen and another is deuterium. As you know these atoms are isotopes and have same atomic number.
Then how can we rank them?
Here comes a special rule. In such situations, we have to consider atomic weight instead of atomic number. So the rule now can be stated as
Since deuterium has atomic weight as 2 whereas hydrogen having 1, the former is given more priority.
Sequence rule 2
Consider the following example.
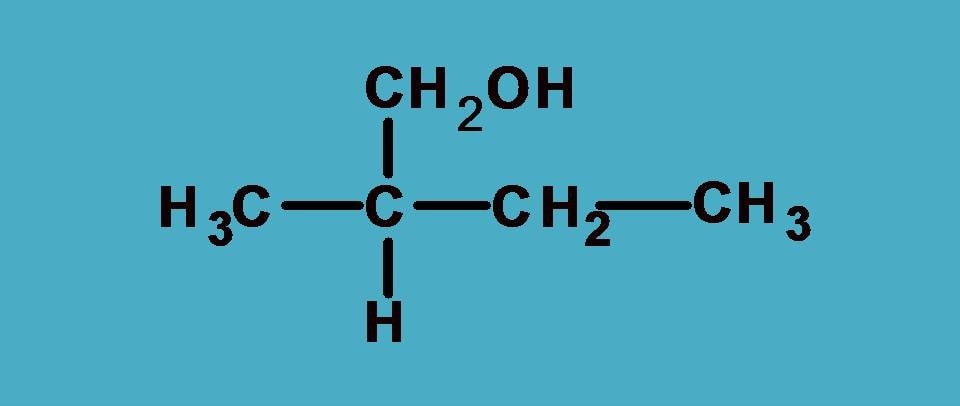
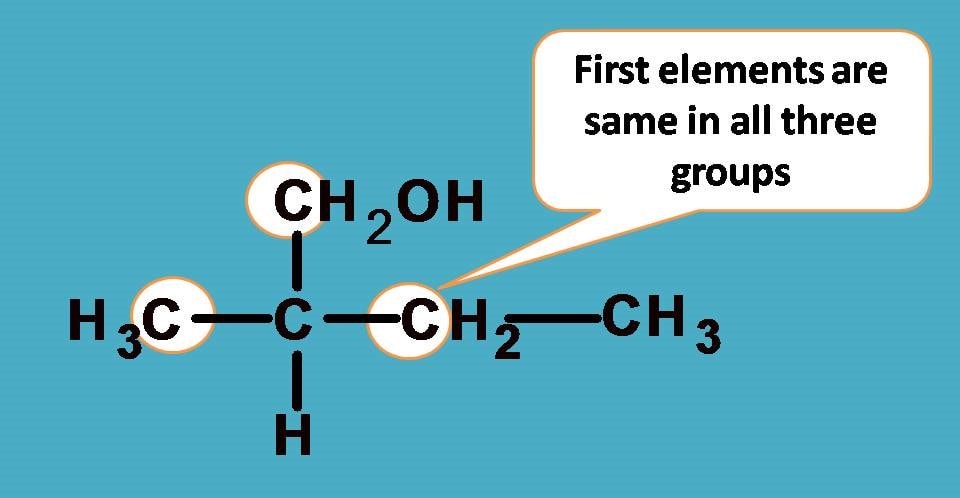
Here you can find the central carbon marked with asterisk is attached to four different groups. One thing is clear that hydrogen is given least preference as it has atomic number as 1. What about other groups? In all other groups the first elements attached to the chiral carbon are same!
How can you give the priority?
So, again we have to search for a new rule. Here it is.
So, simple!
As you see all the first elements are same, let’s go and compare the next elements attached to these first elements and find any point of difference to bring the priority order.
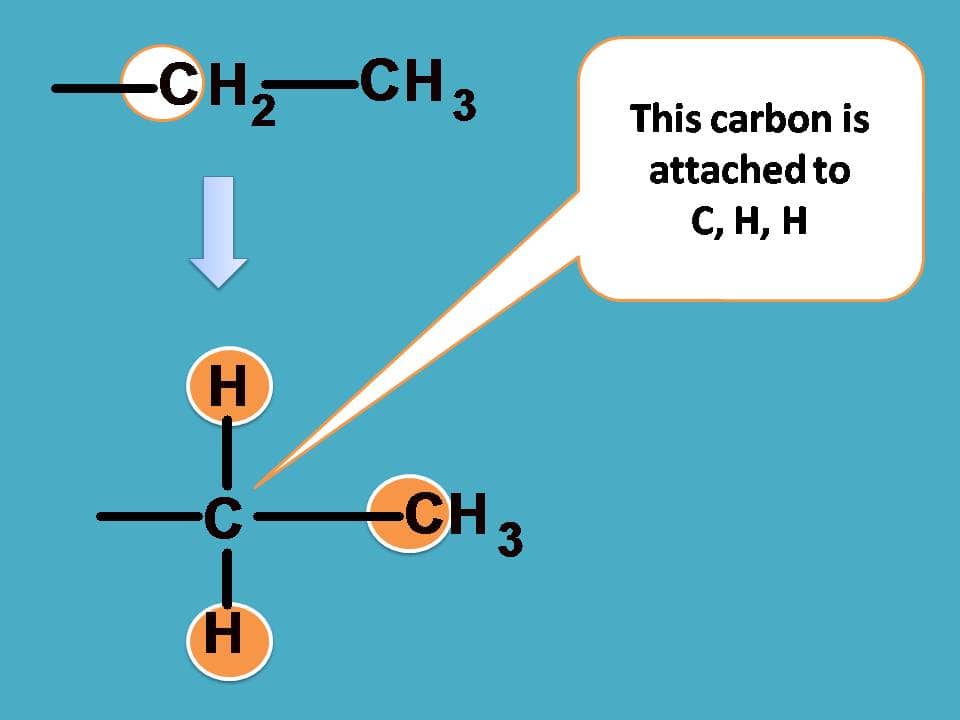
If you consider –CH2CH3 group from the above structure, the carbon of –CH2 is attached with one carbon and two hydrogens.
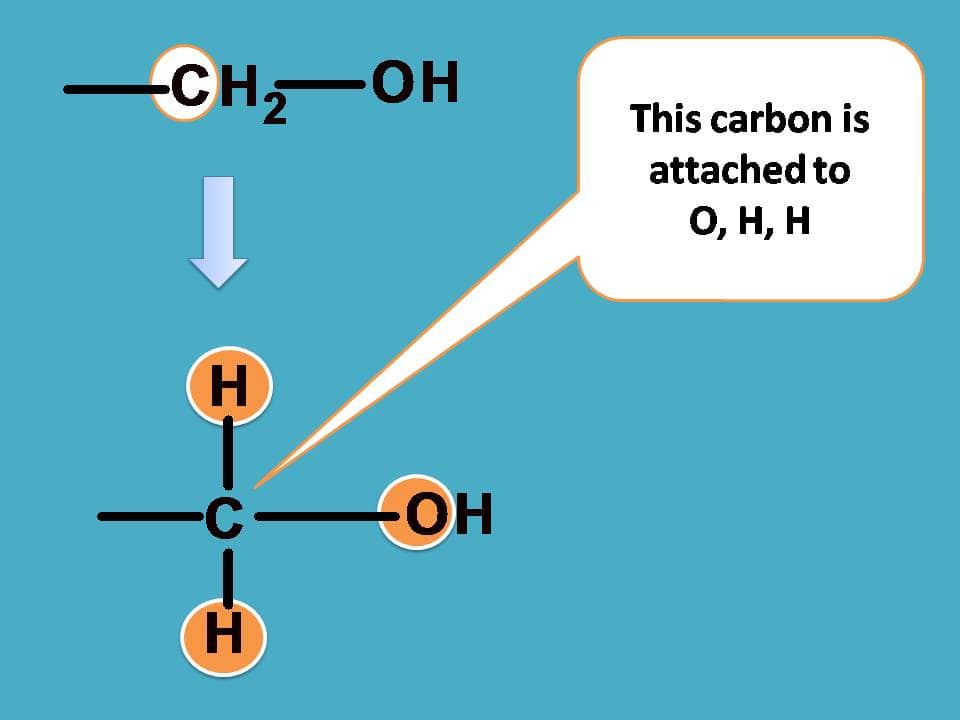
Similarly, for –CH2OH group, the carbon is attached to one oxygen and two hydrogens.
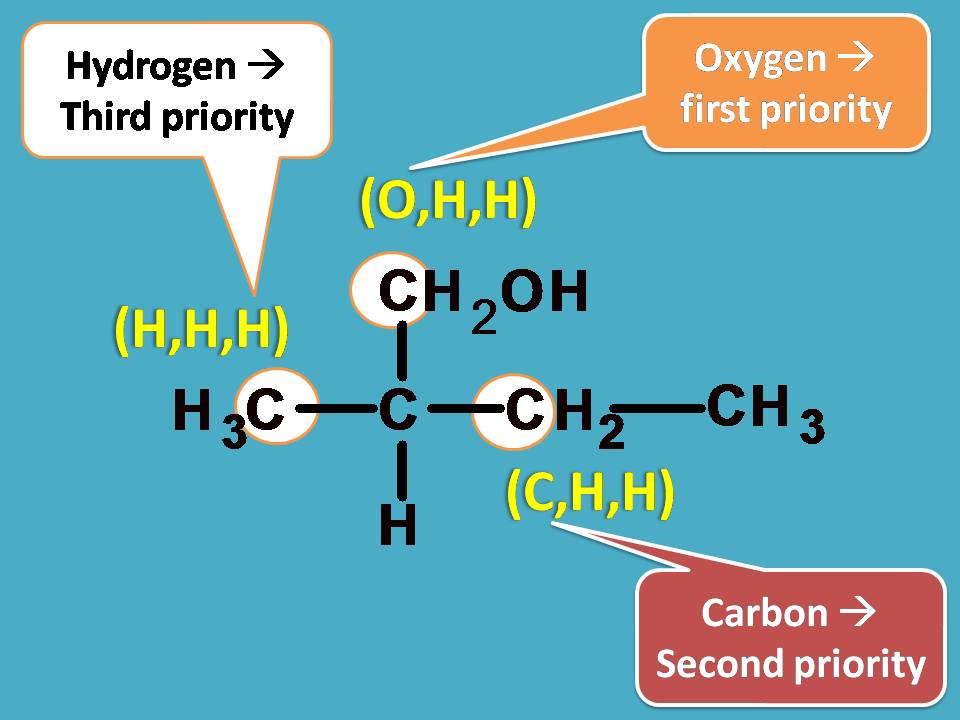
Now, let’s bring all these at one place and find the priority order. You can easily find that –CH2OH group should be given first preference as it contains oxygen with higher atomic number. Next preference is for –CH2CH3 as it contains carbon and finally third preference is for –CH3 as it contains only hydrogens.
Sequence rule 3
We are coming to the end of the story. Let’s take an advanced example.
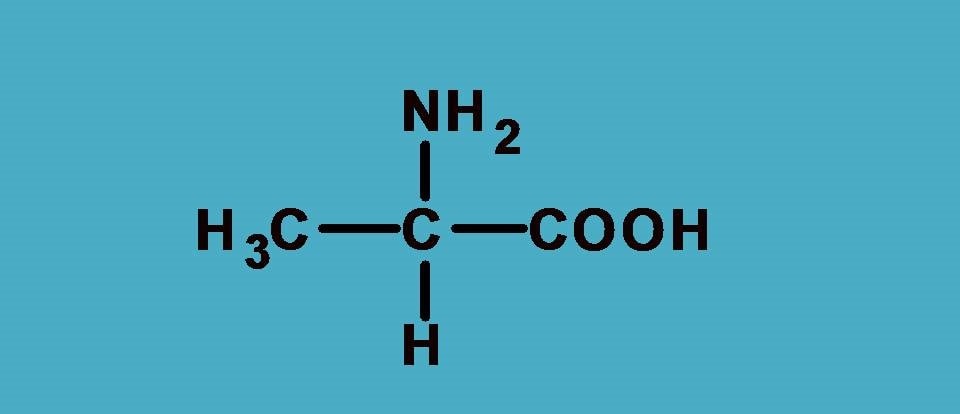
It is a simple amino acid alanine which is optically active due to chiral center. First let’s list out the groups attached here.
–NH2, –COOH, –CH3 and –H
Who secures first rank? Undoubtedly it is –NH2 as the nitrogen has higher atomic number than carbon. That’s fine. Next, there is a tie between –COOH and –CH3 as both starts with carbon. So let’s apply sequence rule 2.
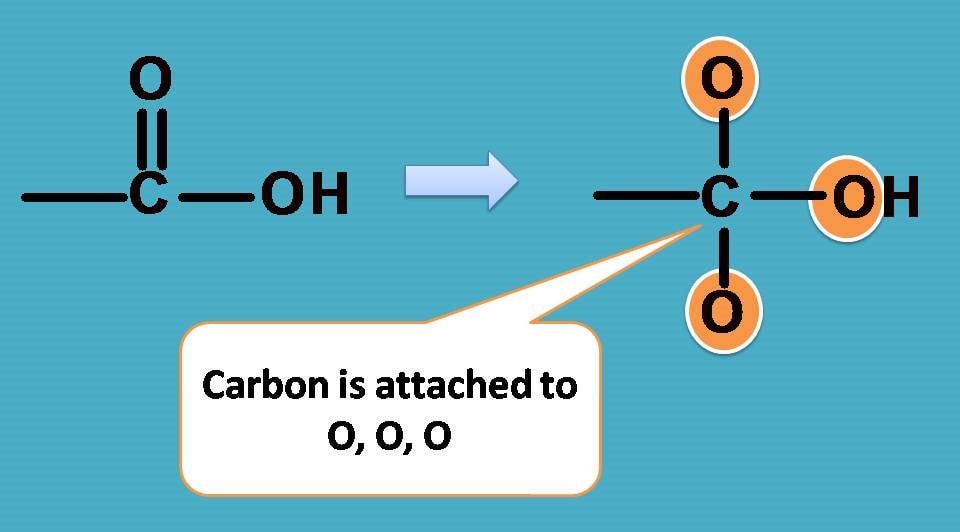
In –COOH, carbon is attached to two oxygen atoms while in –CH3 carbon is attached to three hydrogens.
Is anything looks odd?
Definitely, in –COOH carbon is attached to two elements while in –CH3 it is 3 elements. How can you compare in such unequal situation?
All this is due to double bound in –COOH. Can we consider double bond and single bond as same while giving priority?.
Undoubtedly, they are not same. Here sequence rule 3 stems out.
Assume that a double bond is formed by attachment of element two times.
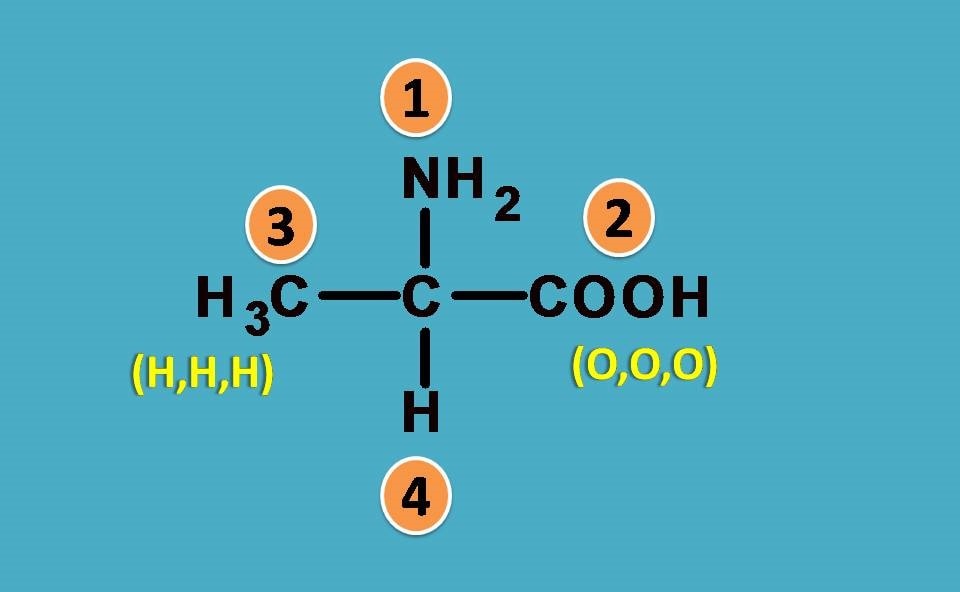
So, simply –COOH is considered as carbon attached to three oxygens. This is a hypothetical concept just used to provide ranking order.
Coming back to the given structure, now we can easily rank the groups. COOH is given more preference over –CH3 as the former attached to three oxygens while later with 3 hydrogens.
So, the final ranking order is
–NH2 > –COOH > –CH3 > –H
Let’s have few more examples by combining all these rules.
Example 1:
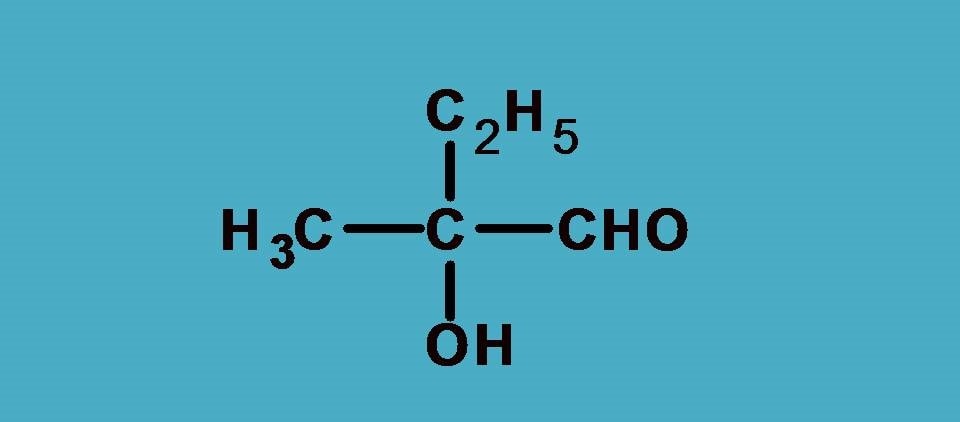
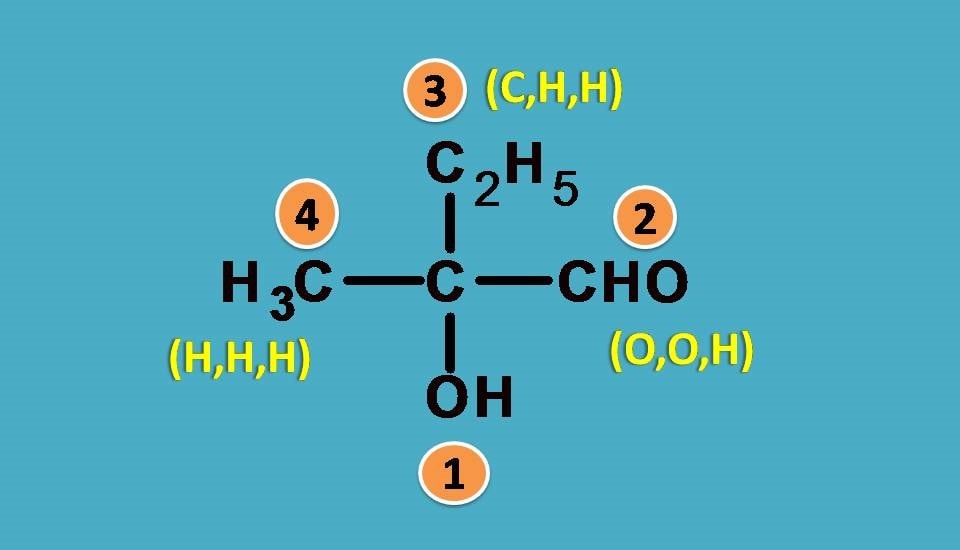
First let’s list out the groups.
–OH, –CHO, –CH3, –C2H5
Obviously , –OH group should be given first preference as it is attached through oxygen. Next preference is for –CHO as the carbon is attached to two oxygens and one hydrogen.
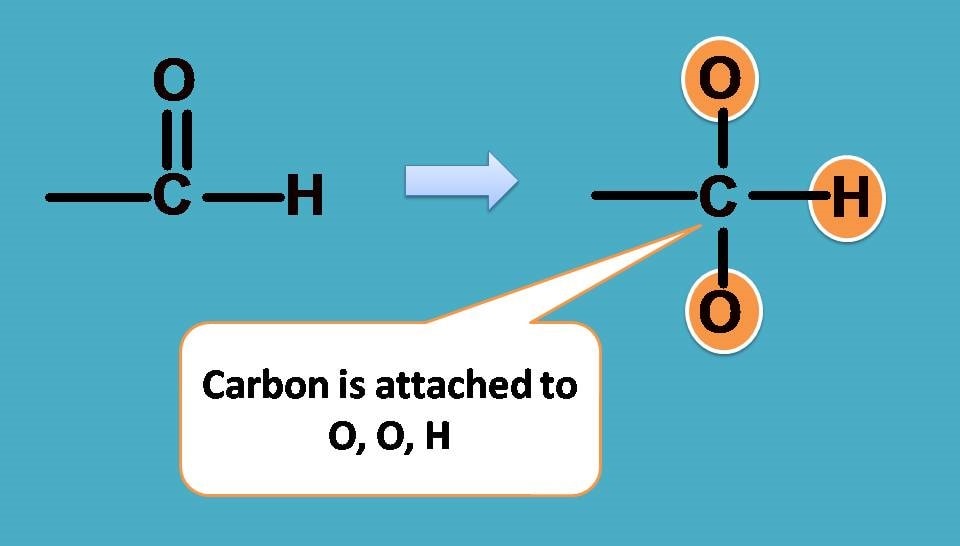
Finally –C2H5 should be given more preference over –CH3 as the former contains carbon connected to one carbon and two hydrogens.
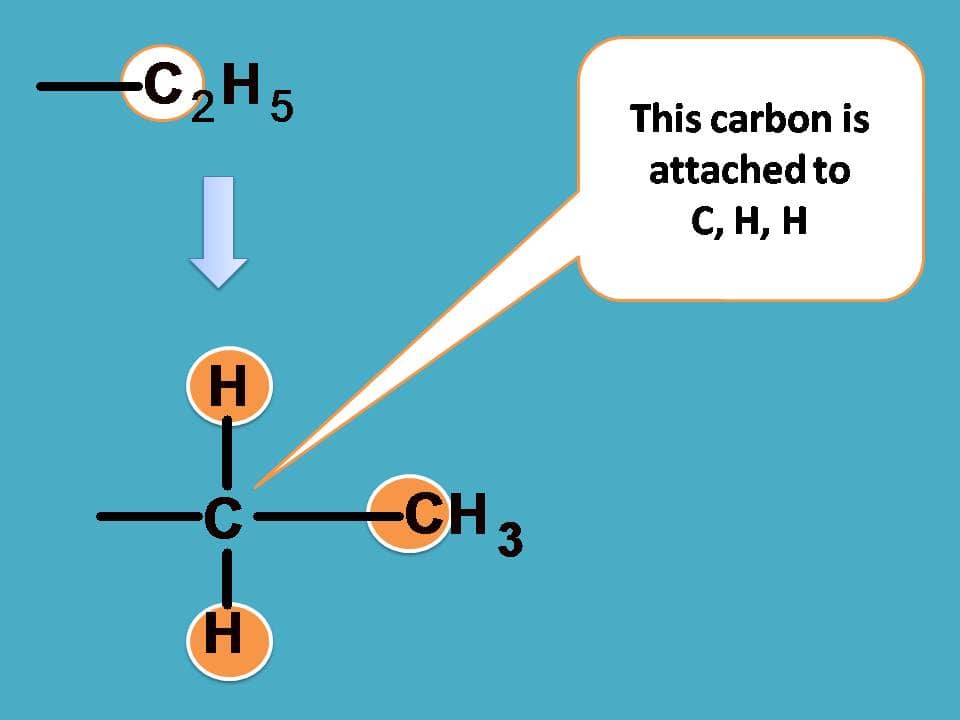
Combining all these the final priority order is –OH > –CHO > –C2H5 > –CH3.
Example 2:
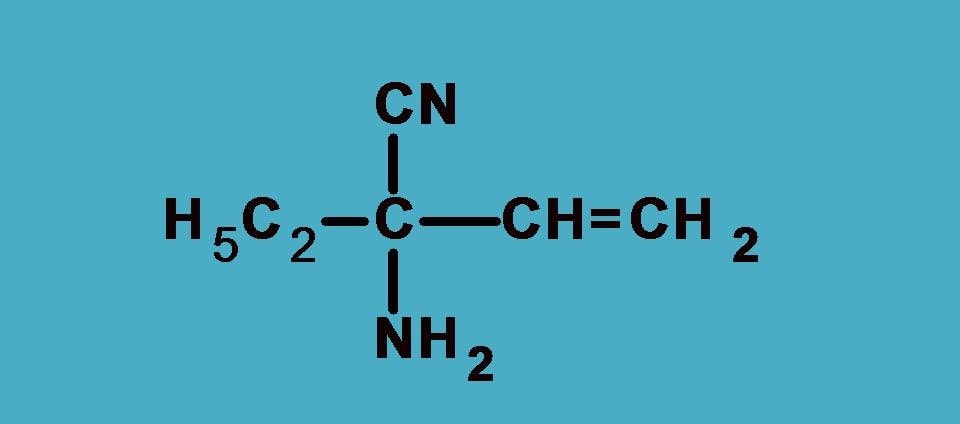
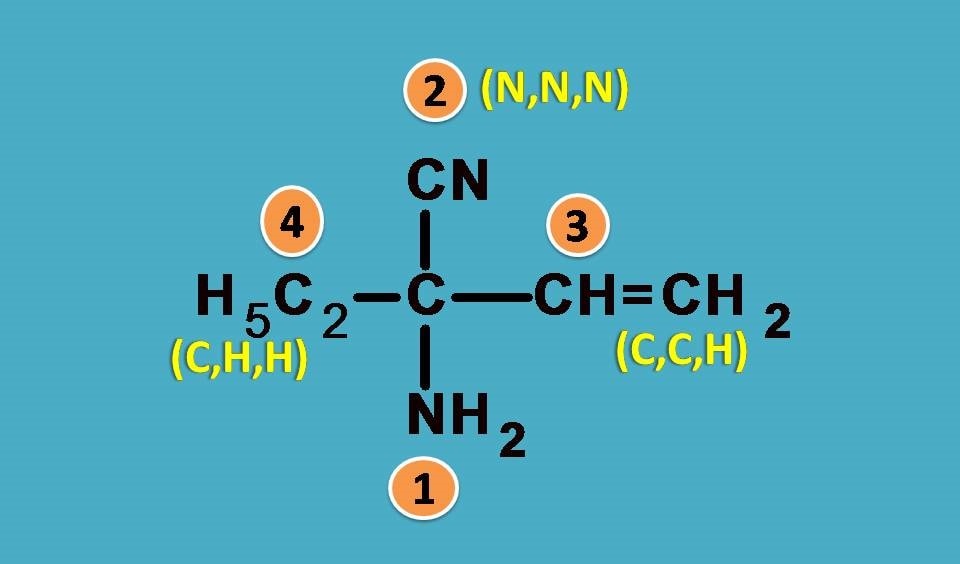
Here the four groups are –NH2, –C2H5, –CN, –CH=CH2
Again NH2 is the first priority group as nitrogen has higher atomic number than carbon. Next we will expand other groups.
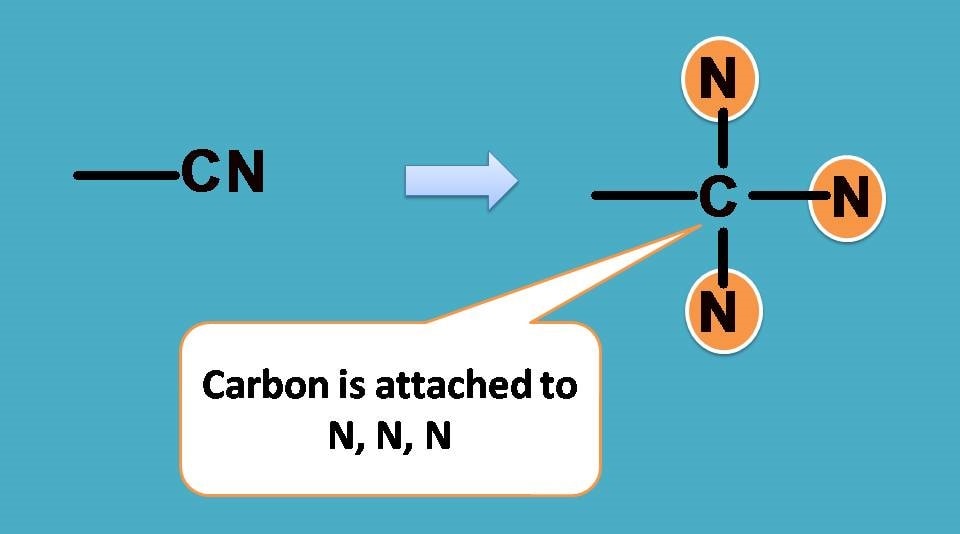
–CN group has carbon attached to nitrogen by triple bond. According to sequence rule 3 we have to consider this group as carbon attached to 3 nitrogen atoms.
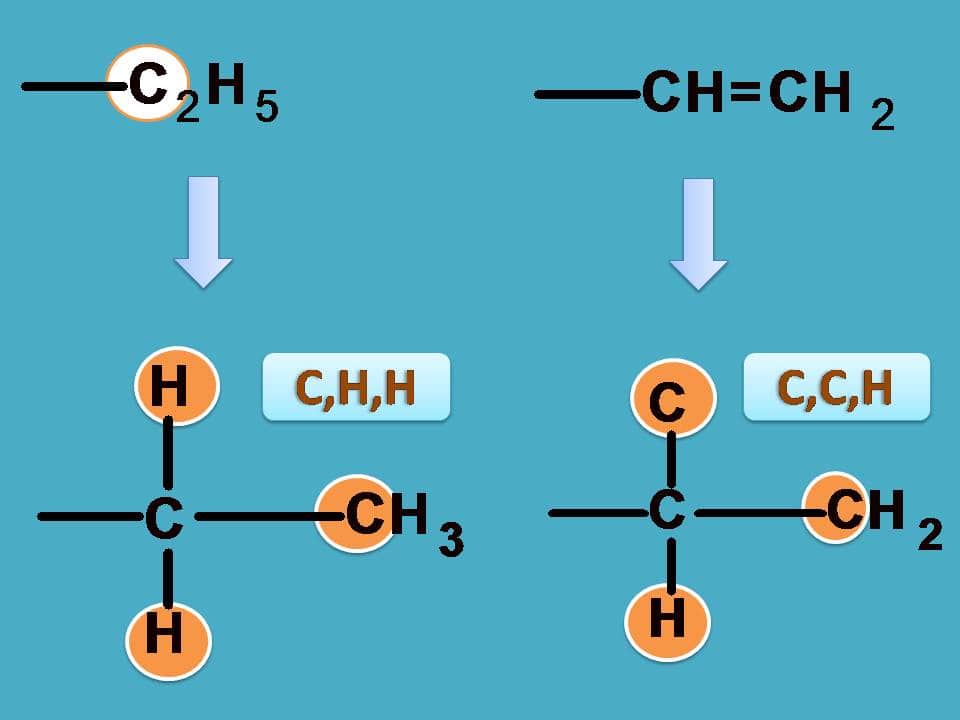
Similarly, –CH=CH2 has carbon attached to two carbon and one hydrogen while –C2H5 as seen in the previous example it is attached to one carbon and two hydrogens.
Combining all, the final ranking order is –NH2 > –CN > –CH=CH2 > –C2H5
Here is a video on how to use sequence rules to provide ranking of groups in order to assign R & S nomenclature.
Conclusion
That’s about sequence rules. These rules are used to provide ranking to the four groups attached to chiral center in order to facilitate labelling the enantiomers with R and S nomenclature. Atomic number is used for ranking and those elements with higher atomic number are given higher ranking. We can extend our approach to the next elements attached when there is a tie in ranking for first elements. Sequence rule 3 is a hypothetical statement that assumes double bonds and triple bonds as multiple single bonds.