Solvents in NMR spectroscopy
by egpat 05 Jun 2019
In any analytical technique a solvent should be selected such that ideally it should not interfere with the study of analyte. A solvent should be easily available, should solubilize the analyte completely, should be inert and of course should not be much expensive.
Still we can have a list of ideal requirements for a solvent but all may not be practically achieved. So we have to select a solvent which matches to maximum significant criteria required for an ideal solvent.
Then, which solvent can be selected for NMR?
Before going to that we should think of what we do with NMR and how solvent can be selected. NMR spectroscopy is a technique that is shown by nuclei having spin quantum number I as ½. So all nuclei may not give NMR signal and few of the nuclei such as 1H, 13C, 19F are active in NMR.
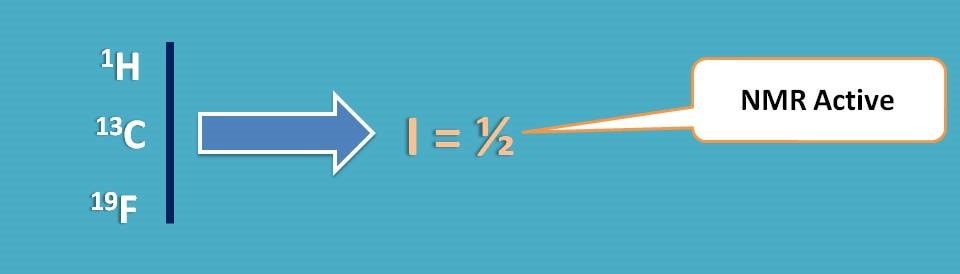
Now we got the clue.
If we select a solvent that doesn’t contain any of the above specified nuclei we can eliminate any interference of solvent on NMR signal of analyte.
That’s nice. Let’s find out suitable solvents for both proton NMR and 13C NMR spectroscopy.
Solvent for proton NMR
Carbon tetrachloride which has only carbon and chlorine atoms is a perfect option here as it is NMR inactive.
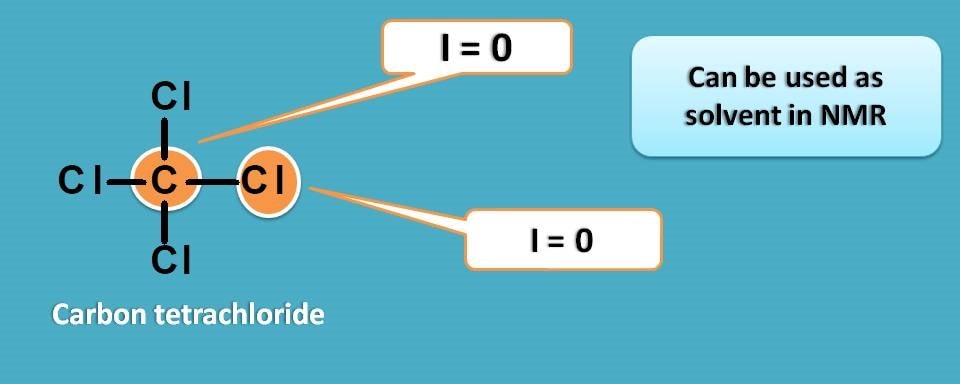
But here we may miss another important required property of a solvent in NMR. Yes, it is solubility. CCl4 is a non polar solvent that can be used with non polar analytes but not suitable for polar samples. So we should search for a polar solvent.
Can we use water as solvent in NMR?
Unfortunately, water has two protons both produce their own NMR signals in proton NMR spectroscopy.
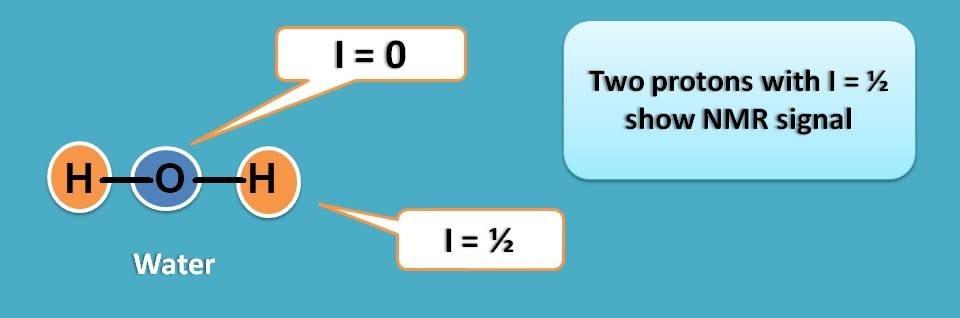
In reality we can’t find any polar solvent without proton as proton is part of most of the polar solvents.
Then what we can do?
Yes, we have another option here. As we have discussed earlier, NMR spectroscopy is not shown by all the nuclei and nuclei with spin quantum number, I, not equal to ½ are NMR inactive. This gives advantage in differentiating isotopes in NMR.
For example proton has I=½ whereas its isotope deuterium has I=0. So if replace proton with deuterium we can get a solvent free of interference.
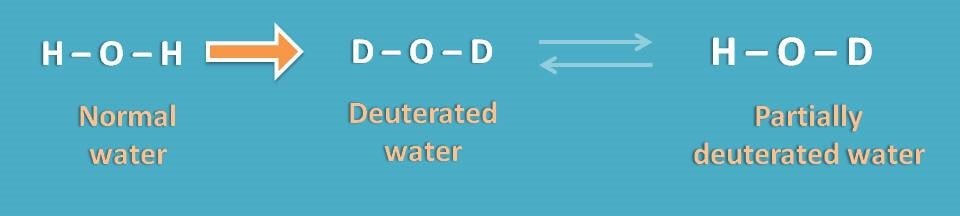
This brings the list of deuterated solvents in proton NMR spectroscopy. First lets replace the protons in water molecule with deuterium to get deuterated water.
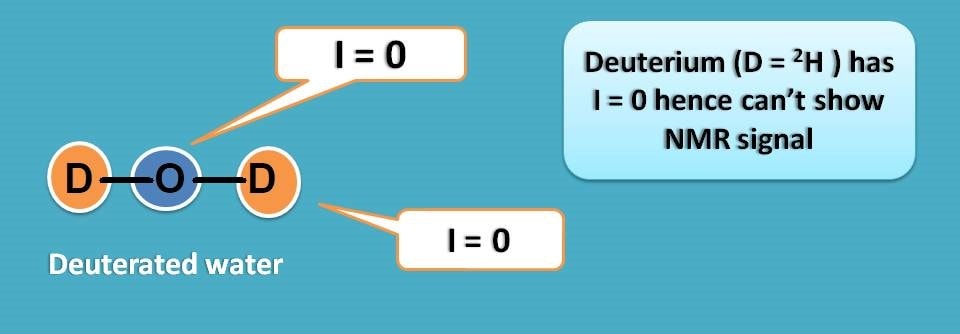
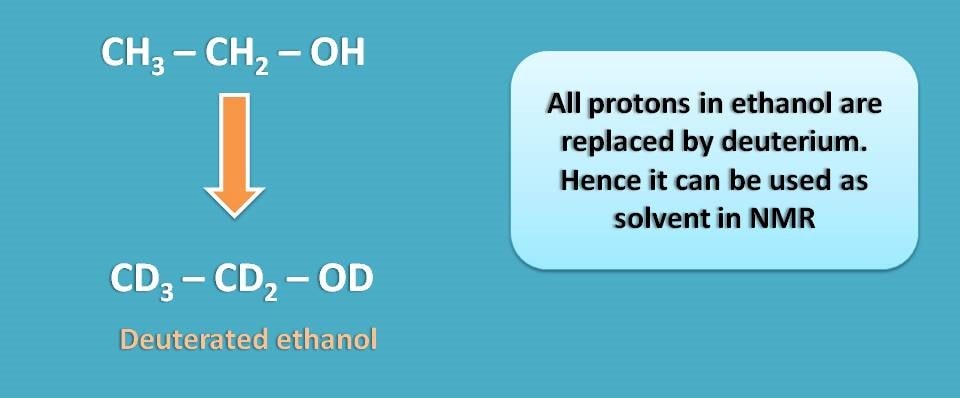
Similarly, in ethanol all the five protons can be replaced with deuterium to get deuterated ethanol.
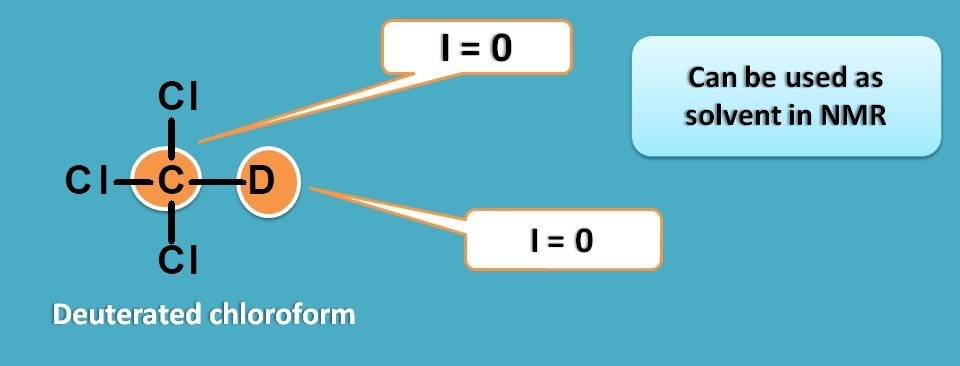
Chlorofom is generally acts as good solvent and when it is deuterated it can be used as solvent in proton NMR.
Similarly we can use other deuterated solvents as listed below.

Even deuterated solvents are used in NMR, they can’t be completely free of NMR signals that may interfere with study. For example, if we prepare Deuterated water, we can also find a trace amount of HOD in the solvent where one the deuterium is again exchanged for proton.
Similarly, if we prepare deuterated chloroform, still we can find trace amount of chloroform in the solvent.
Solvent with proton
In proton NMR spectroscopy, we can also use trifluoro acetic acid as solvent. Surprisingly, even it has a proton in the carboxylic acid group still it can be used as solvent.
How it is possible?
It's all depends on the chemical shift range we going to study. The proton in trifluoro acetic acid has high deshielding effect due to electron withdrawing carbonyl group. So it falls at the extreme of NMR spectrum table with a chemical shift ranging between 9-12 ppm.

Suppose we are studying an analyte that shows NMR signals below 9 ppm we can simply select the trifluoroacetic acid as solvent as its signal falls out of the range of study.
Solvent for 13C NMR
Now let’s see which type of solvent can be selected for 13C NMR. Unlike proton NMR, here solvent may contain carbon. Yes, that’s all due to again isotopes of carbon.
Normal 12C carbon has spin quantum number I=0 therefore it is inactive in NMR. So we can easily select any solvent that has carbon and no need to replace it.
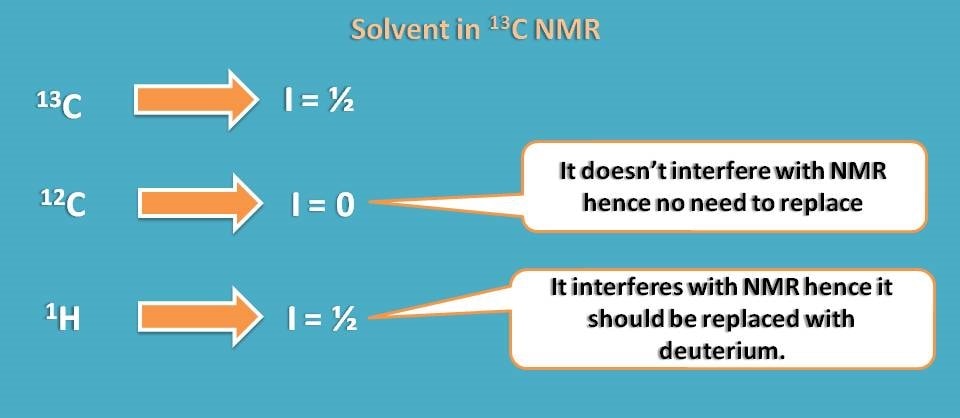
But again proton may interfere with study, so we can again select deuterated solvents.