What is chemical equivalence and magnetical equivalence in NMR?
by egpat Posted on 08-06-2017
Chemical and magnetic equivalence plays important role in determining the number of peaks in NMR spectroscopy. Protons in an organic compound may have different environment either by linkage or by orientation resulting in non-equivalence. All nuclei which are magnetically equivalent will give single NMR signal. Similarly nuclei which are magnetically non-equivalent will produce different NMR signals.
To learn more about number of peaks in NMR,see how to determine the number of NMR peaks
One of the interesting fact is that if two protons are magnetically equivalent then they will be chemically also equivalent. But the reverse is not true. That means, if two protons are chemically equivalent then it may not always indicate that they are magnetically equivalent. Sometimes they may be non-equivalent magnetically even they are chemically equivalent. So let's discuss how this is possible, what is chemical and magnetic equivalence along with examples.
Chemical equivalence
Let's start with chemical equivalence.
Chemical equivalence means similar chemical environment. When nuclei are attached by similar linkage they are called as chemically equivalent.

Let’s take an example of methane.
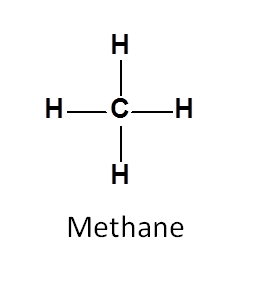
In methane, protons has spin quantum number I=1/2 and herefore can give NMR signal. Here all the protons are attached to carbon in similar way and hence chemically equivalent.
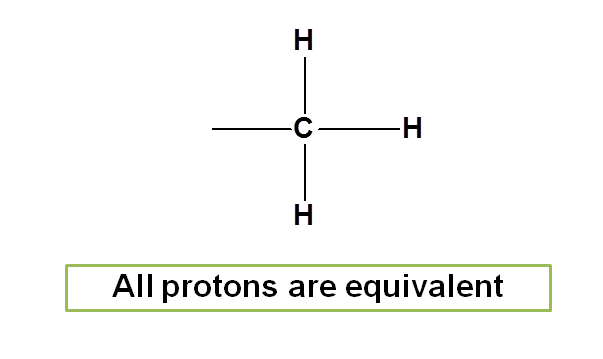
In methyl group all protons are equivalent as they attached similarly to the carbon.
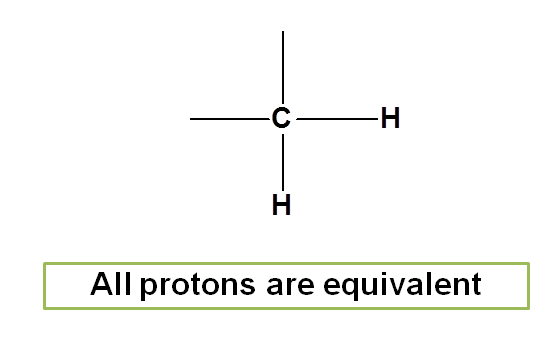
Similarly in methylene group the two protons are equivalent.
Now let’s take another example. Consider 1-bromopropane.
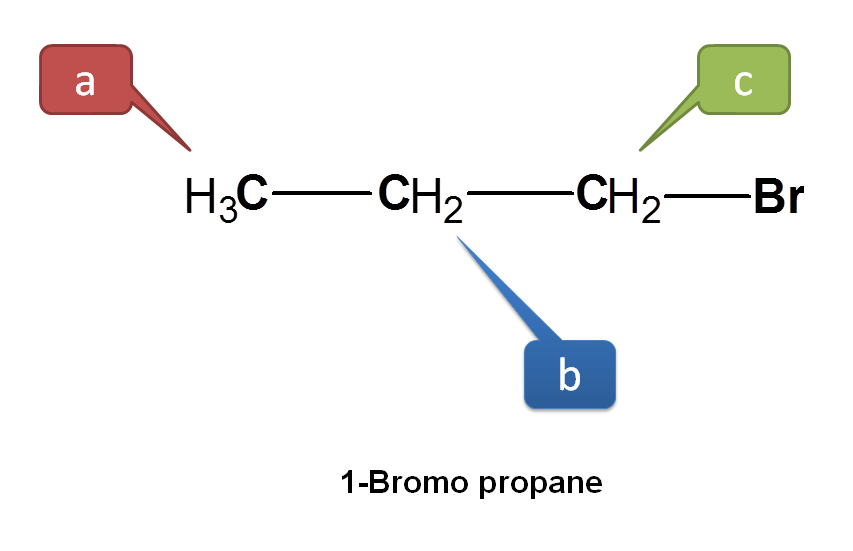
Here CH3 protons are not equivalent with other protons hence indicated by ‘a’. Now we can see two types of –CH2 protons. Protons indicated by green color are not attached in similar way as the protons indicated by blue color. The protons indicated by green color are attached as – CH2Br whereas those with blue color as –CH2-. Hence all these protons are equivalent.
Now consider 2-chlorobutane.
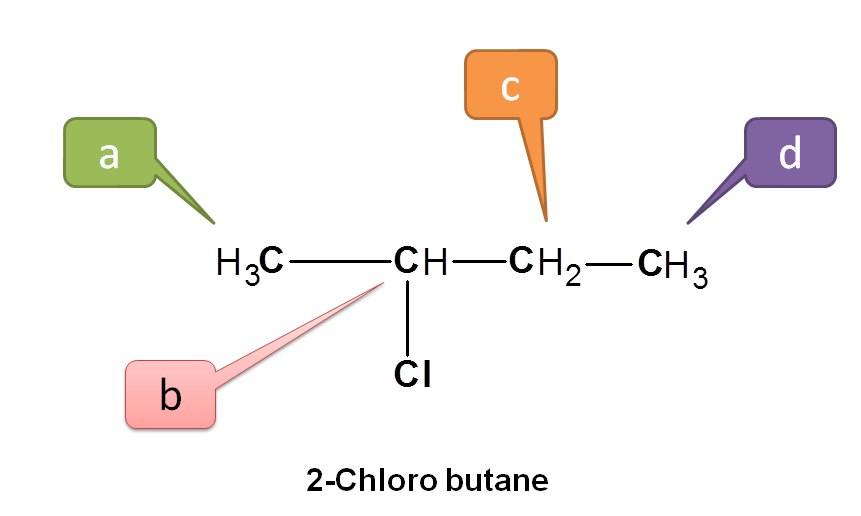
Here CH3 indicated by blue and green color are not equivalent. Protons indicated by green are attached to –CHCl- where as protons indicated by blue are attached to –CH2-. Similarly protons –CHCl- and –CH2- are also non-equivalent. Hence all the protons above are non-equivalent.
Magentic equivalence
When nuclei are arranged in similar way in the space they are called as magnetically equivalent.
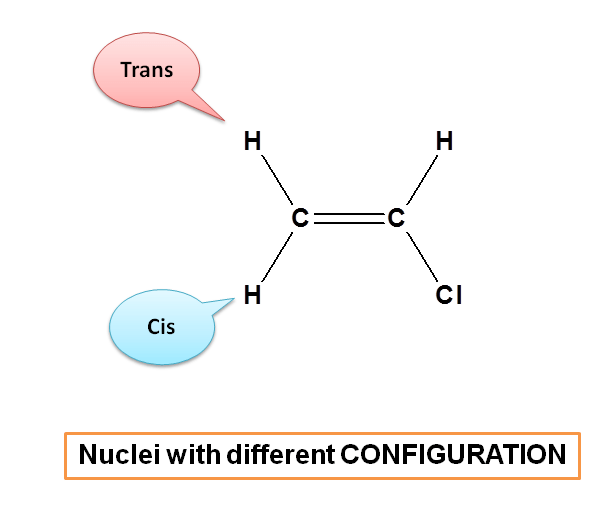
Here protons present in cis and trans position have similar linkage but different configuration. cis-proton on the same side whereas trans-proton on opposite side of the double bond. Hence these protons are chemically equivalent, but magnetically not equivalent.
Compounds will have different configuration when they exhibit restricted rotation. Alkenes and cyclic compounds show restricted rotation hence may show magnetic non-equivalence.
Let's consider vinyl bromide.
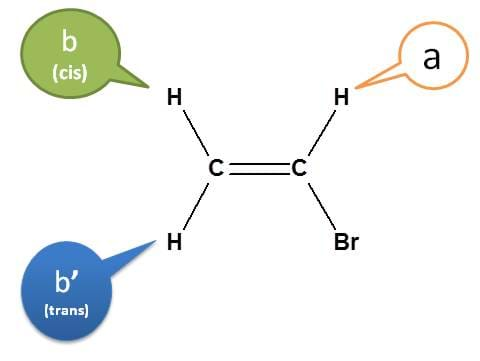
In vinyl bromide, the protons of –CH2– are not magnetically equivalent. The proton indicated by green is cis and blue is trans to other proton on -CHBr.