- Home >
- Test papers >
MCQ on acid-base titrations: Page-3
The reagent used in kjeldahl method is
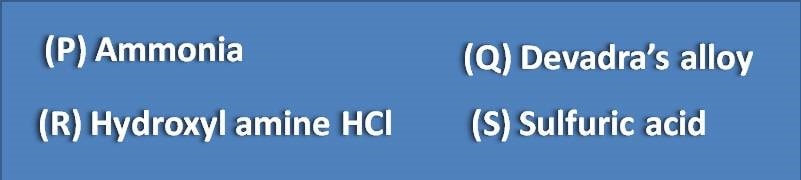
(A) P
(B) Q
(C) R
(D) S
The pH of 0.01 M NaOH is
(A) -2
(B) 2
(C) 12
(D) 10
Hydroxyl value is defined as
(A) No. of mg of KOH required to neutralize the acetic acid liberated from the acetate ester of analyte
(B) No. of mg of KOH required to neutralize the oxalic acid liberated from the oxalic ester of analyte
(C) No. of mg of KOH required to neutralize the benzoic acid liberated from the benzoic ester of analyte
(D) No. of mg of KOH required to neutralize the steraic acid liberated from the stearate ester of analyte
Which of the following can be determined by kjeldahl procedure

(A) Alkyl cyanides
(B) Potassium nitrate
(C) Proteins
(D) Azo compounds
The nature of weak base with pKb value 4.5 at pH of 10.0 is
(A) Salt form
(B) Acidic form
(C) Neutral form
(D) Basic form