- Home >
- Test papers >
MCQ on NMR spectroscopy: Page-1
Identify the nuclei that has spin quantum number I=0 from the following.
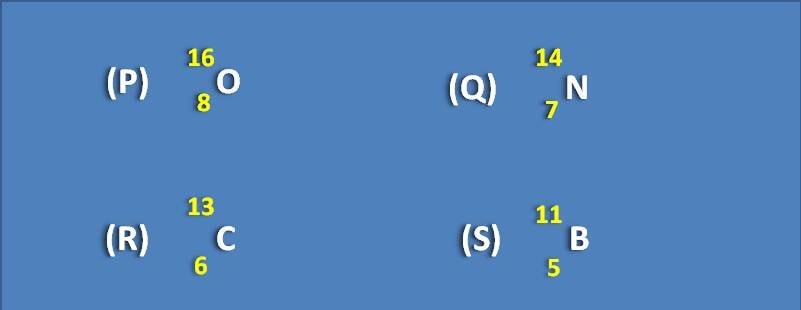
(A) P,S
(B) Q,R
(C) P
(D) Q
Nuclei with odd number of protons and odd number of neutrons will have spin quantum number as
(A) 1/2
(B) 3/2
(C) 0
(D) 1
The number of peaks possible in proton NMR for the following compound is
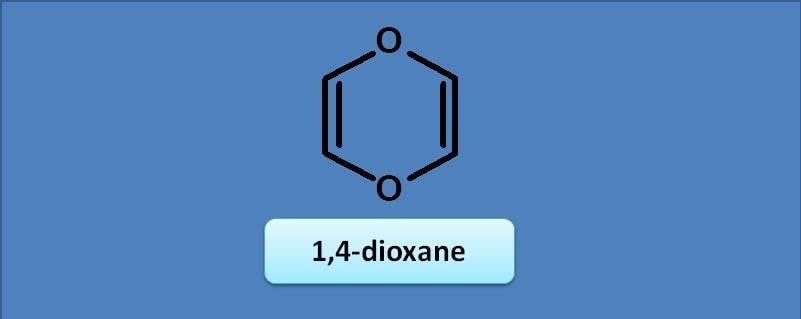
(A) 2
(B) 3
(C) 1
(D) 4
The possible spin states of a nucleus with spin quantum number 1/2 are
(A) 0
(B) 1
(C) 2
(D) 4
The unit for magnetic moment is
(A) Tesla
(B) Gauss
(C) Joule/gauss
(D) No units