- Home >
- Test papers >
MCQ on poentiometry: Page-3
The electrochemical analysis which measures the current generated at the electrode in an ionic solution by application of potential is known as

In titration of iodine with sodium thiosulfate, the equivalent weight of sodium thiosulphate is ( Mol Wt 248)
(A) 248
(B) 124
(C) 62
(D) 24.8
Calculate logarithm of the equilibrium constant of following redox cell
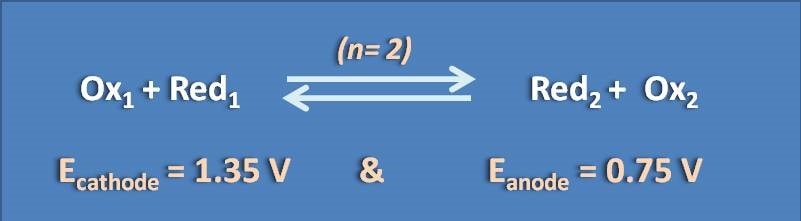
(A) 10
(B) 15
(C) 20
(D) 30
How many grams of sodium thiosulphate should be taken to prepare 100 ml of 0.1 N solution
(A) 8 gm
(B) 1.24 gm
(C) 2.48 gm
(D) 12.4 gm
How many grams of sodium thiosulphate should be taken to prepare 100 ml of 0.1 M solution
(A) 8 gm
(B) 1.24 gm
(C) 2.48 gm
(D) 12.4 gm