- Home >
- Test papers >
MCQ on Principles of titrimetric analysis: Page-4
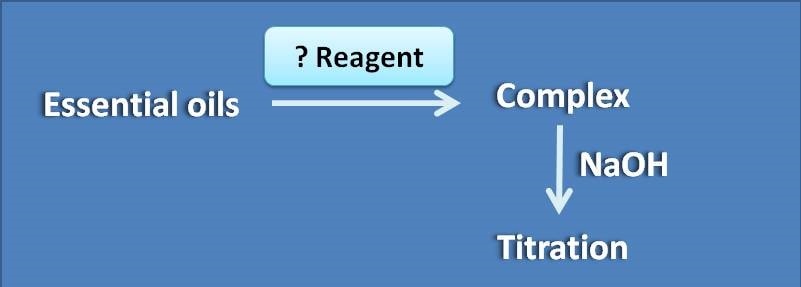
(A) Sulphuric acid
(B) Hydroxyl amine hydrochloride
(C) Eosin
(D) Hydrazine
(A) A broad range of end point
(B) A narrow range of end point
(C) Chain reactions
(D) Approximate pH of the solution
(A) Stoke’s equation
(B) Henderson-Hesselbalch equation
(C) Mosley’s equation
(D) Raoult’s equation
(A)The rate of reaction is proportional to the product of the active masses of the products
(A) The rate of reaction is proportional to the product of the active masses of the reactants
(A)The rate of reaction is proportional to the sum of the active masses of the reactants
(A)The rate of reaction is proportional to the product of the active masses of the products
(A)The product concentration is equal to reactant concentration
(B)The rate of forward and backward reactions are zero
(C)The rate constants of both forward and backward reaction are same
(D)All of the above