Mechanism
You can observe that acetylcholine structure has two important moieties such as
- Cationic group
- Ester group
Correspondingly, acetylcholinesterase has two active sites that are involved in binding and cleavage of acetylcholine. They are
- Cation binding site
- Ester binding site
Cation binding site is made up of glutamate having carboxylate anion hence negative in charge. Therefore it attracts positively charged quaternary ammonium group on the acetylcholine and responsible for its binding by ionic interaction.
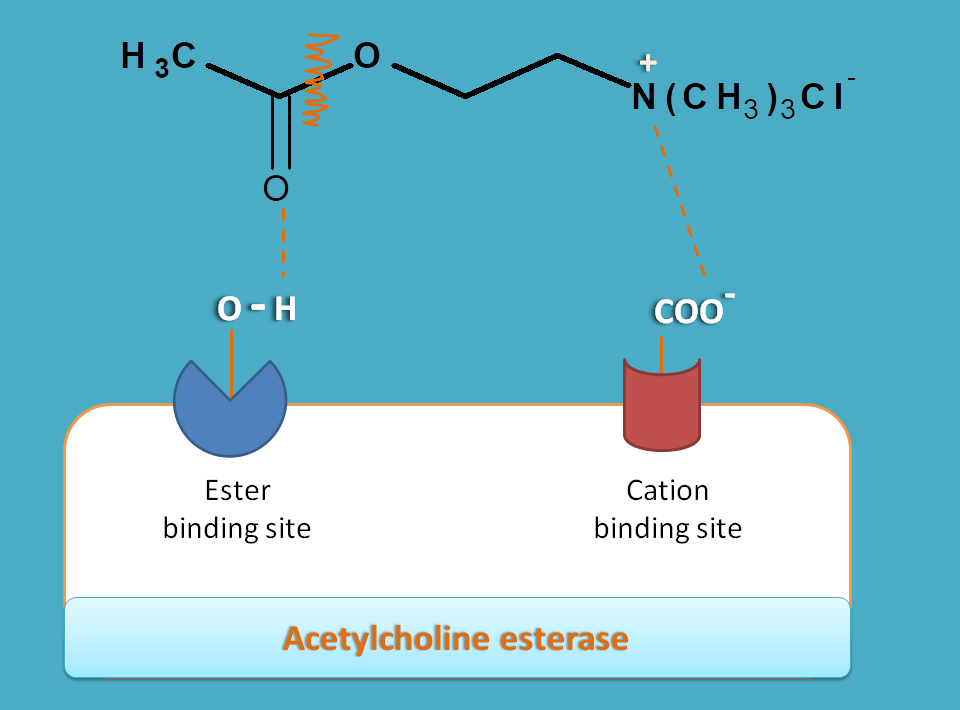
On the other hand, ester binding site, also called as esteratic site, is responsible for cleavage of acetylcholine at ester group leading to formation of choline and acetate. This site is made up of serine having hydroxyl group catalysed by histdine. This hydroxyl group can interact with ester group in acetylcholine by hydrogen bonding and cleaves it to choline.
Which site is important for inhibition?
Obviously from the above discussion it is clear that cation binding site is only responsible for binding whereas ester binding group is responsible cleavage which is more important and hence it should be inhibited.
Many of the anticholinesterases bind to both the sites thereby inhibit its activity while organophosphates mainly bind to esteratic site with no action on cation binding site, still equally active. his indicates that cation binding site is not essential for the drugs to show their activity.
So, we discuss further how these drugs interact with ester binding site and show their action.
Short acting
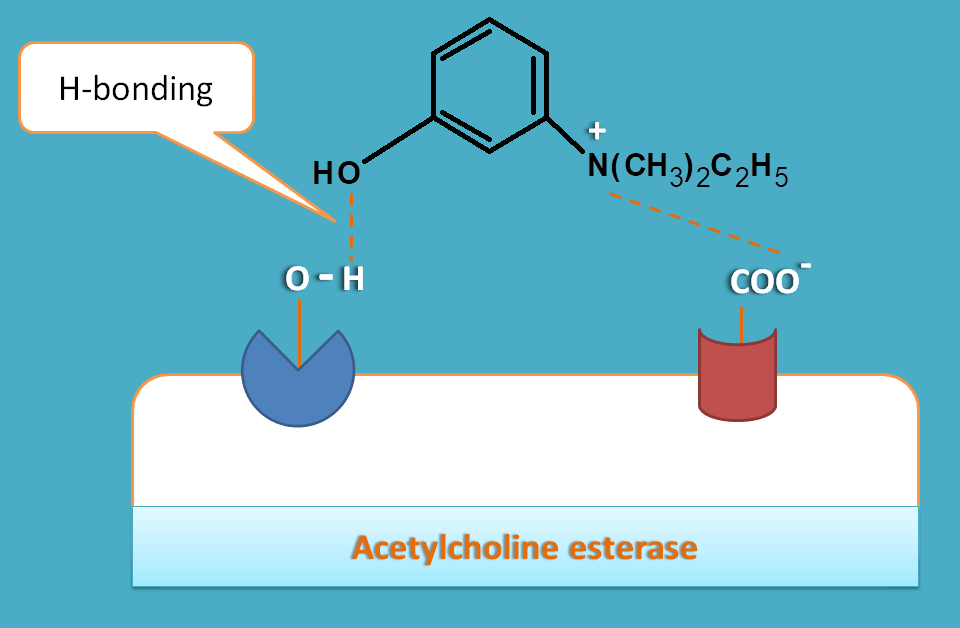
One of the drug in this category is edrophonium. This drug has simple hydroxyl group which forms a hydrogen bond with serine group at ester binding site. This prevents interaction of acetlycholine with serine residue hence its cleavage is inhibited.
Medium acting
All these drugs have a common structural moiety, carbamate. This group is transferred to serine hydroxyl group thereby it is prevented.
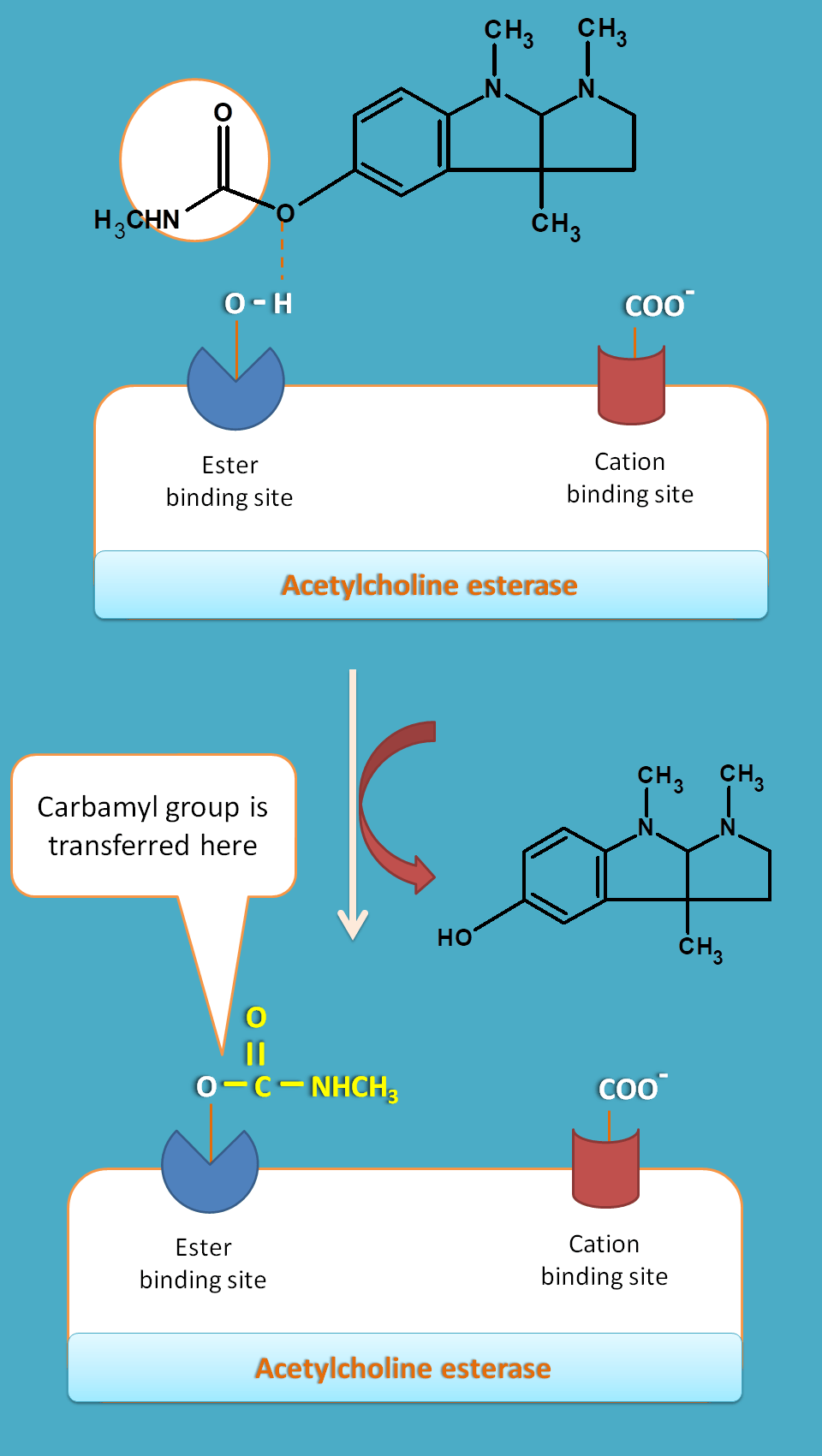
Medium acting drugs act by carbamylation
Long acting
Long acting drugs like organophosphates have phosphorous group which interacts with hydroxyl group of serine residue thereby prevents acetylcholine to act. As phosphorylation is irreversible process these drugs produce long term inhibition of the enzyme.
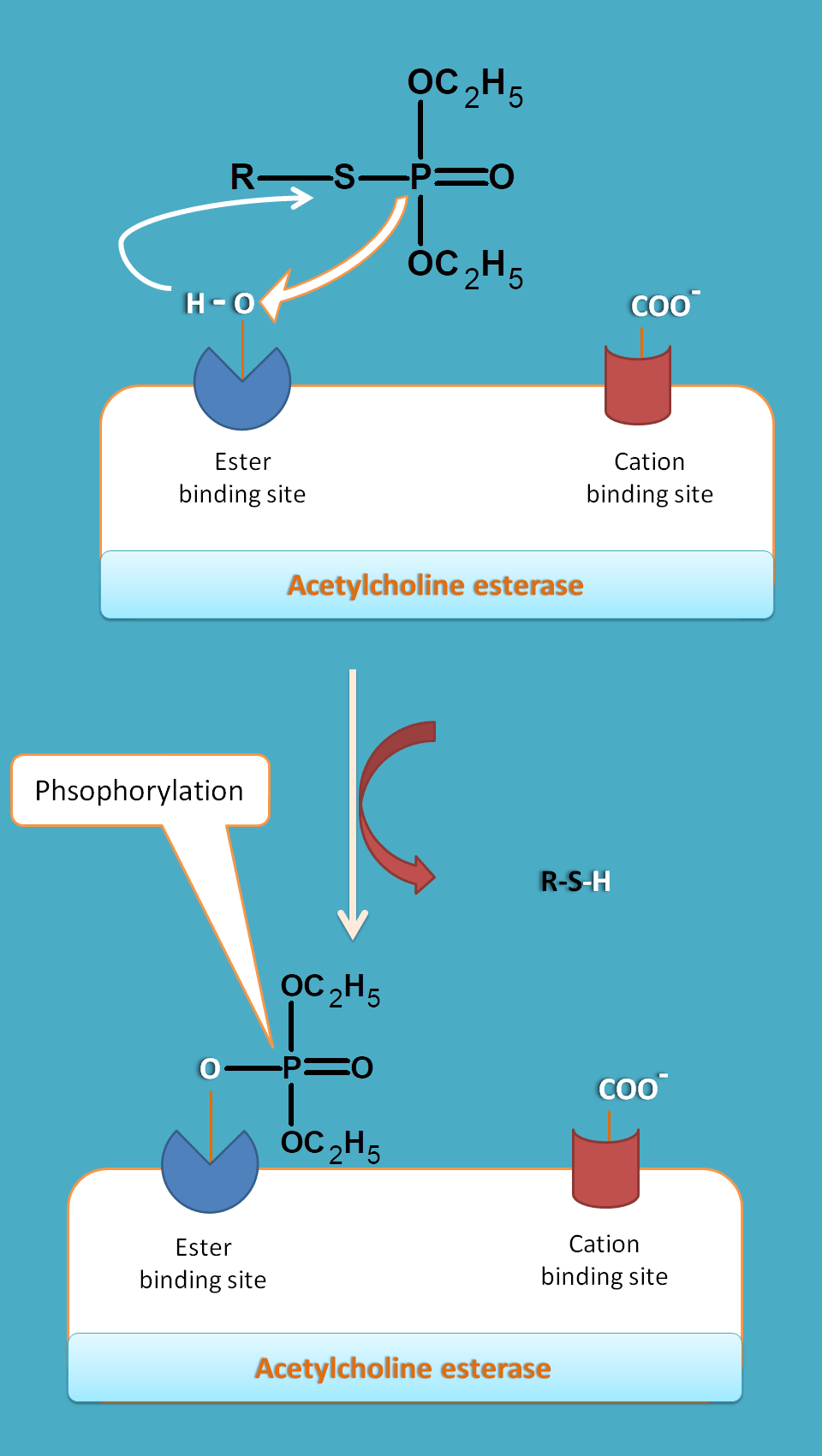
Long acting drugs act by phosphorylation.
The relative strengths of the interaction follow the below order
Hydrogen bonding <Carbamylation <<phosphorylation
Hydrogen bonding is weakest and phsophorylation is the strongest bond with carbamylation as intermediate. Since cholinesterase inhibitors interact by these three mechanisms, they also show same pattern in their duration of action.
Short acting < Medium acting < Long acting
Short acting drugs form weak hydrogen bond whereas long acting drugs form phosphorylation which is irreversible.