How to determine the number of peaks in proton NMR
by egpat 09-06-2017
The number of peaks shown by a compound in proton NMR depends on the presence of magnetically non-equivalent protons in the compound. So in order to predict NMR spectra, we should count protons with different magnetic environment.
Protons may differ in chemical and magnetic equivalence due to their difference in both attachment and arrangement in the space. Here we will see few examples and how we can determine the possible number of peaks in proton NMR for each compound. Let’s start with simple structures.
Compounds with different linkage
Organic compounds mainly contain different protons attached at different locations to the parent chain. All these protons are chemically non-equivalent and therefore give different signals NMR spectroscopy .
Step 1: Identify the protons with different linkage
Initially observe the structure for protons with different linkage. Protons with same linkage are considered as similar type .
Example 1:

Here all protons are equivalent as they are similarly connected to carbon. Hence the number of NMR signals is 1.
That’s so simple as we really count different types of protons present in a compound to assess the number of peaks in proton NMR. Then what about the solvent used in NMR? They may have protons which may give their own peaks in NMR spectra. Exactly, it’s true and that’s why we have to select a solvent in NMR that is devoid of protons. Practically this can be achieved by deuterated solvents where all the hydrogens are replaced by deuterium.
Example 2 :
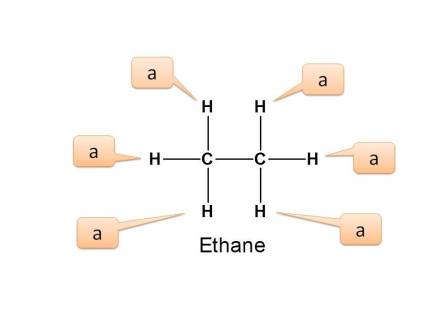
In the above example, all the six protons are equivalent as they are connected in similar way to carbon which is connected to another carbon. Here all these protons are primary protons.
Hence again the number of NMR signals is 1.
Example 3 :
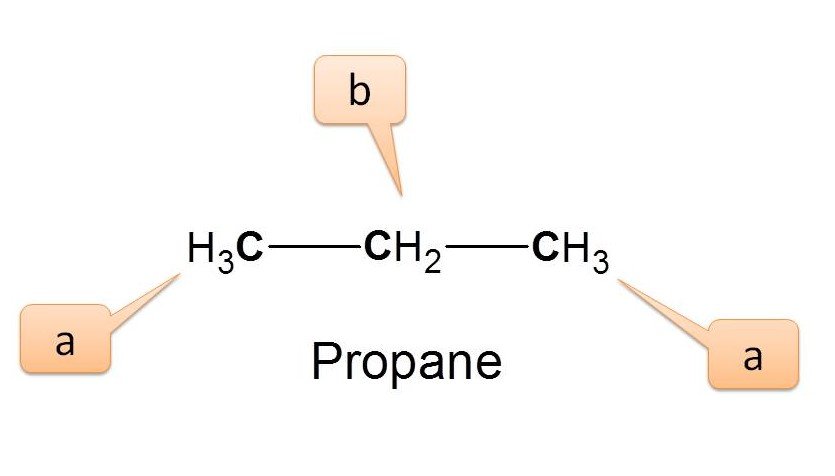
Now let’s go to the propane.
Here we can observe two types of protons viz. methyl (-CH3) protons and methylene (-CH2) protons indicated by ‘a’ and ‘b’ respectively. The terminal methyl groups are equivalent and labelled as ‘a’. Hence the number of NMR signals is 2.
Since these protons are chemically not equivalent, they will show different chemical shifts chemical shifts in NMR spectra. Sometimes we can easily identify the type of proton based on their chemical shift by comparing the value in NMR spectrum table . In the above example, if a proton shows a chemical shift of 0.9 ppm it will be due to methyl proton while proton with 1.3 ppm as chemical shift will be of methylene proton.
Compounds with different configuration
Configuration is the relative arrangement of the atoms or groups within the space. Till now, we have seen simple examples where protons differ by linkage only. These compounds have single configuration in the space.
On the other hand compounds that can show stereoisomerism can exhibit different configuration. Practically many of the important organic and medicinal compounds are unsaturated and therefore show different configuration. Similarly cyclic compounds show geometrical isomerism hence protons lie in different environment.
- See here to know more about NMR signals in cyclic compounds
Now let’s see few examples of aliphatic unsaturated compounds and how to predict NMR signals.
Let's take simple example of vinyl chloride.
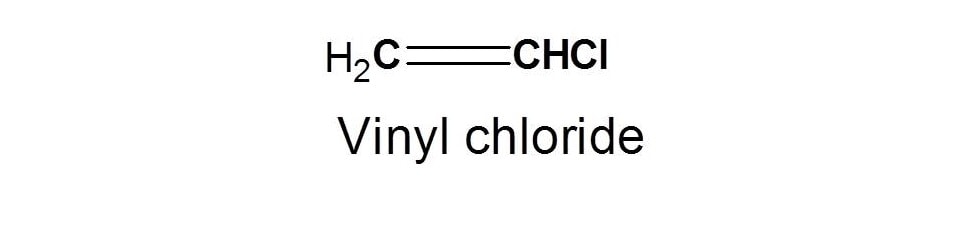
In the above structure, we can observe two types of protons with different linkage viz. Vinylic (CH2=) proton and=CHCl. These protons are indicated by letters a and b respectively.
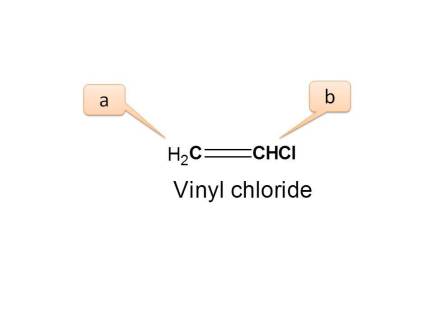
If we expand the structure and observe its configuration, we can observe three types of protons.
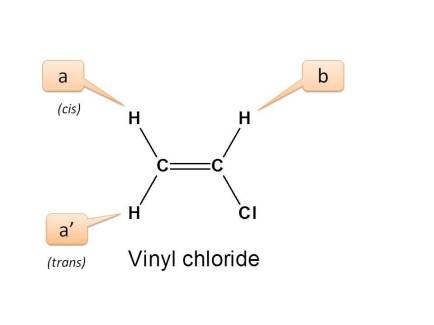
- Proton indicated by a is trans to “Cl” group
- Proton indicated by a ’ is cis to “Cl” group
- Proton indicated by b is adjacent to “Cl” and attached to same carbon.
Here the protons present on=CH2 are connected equally and they are chemically equivalent.
But they have different configuration hence magnetically non-equivalent.
- Hence the total no. of NMR signals possible is “3”
Now indicate all the magnetically non-equivalent protons with letters a,b,c …etc. If protons have same linkage but different configuration they are differentiated by letter prime.
This is already observed in above examples where each magnetically non-equivalent nuclei is indicated by alphabetic letter.
Now count all protons which give total no. of NMR signals possible.
Working examples
Let’s apply the above steps with few working examples.
Working Example 1: Isobutane
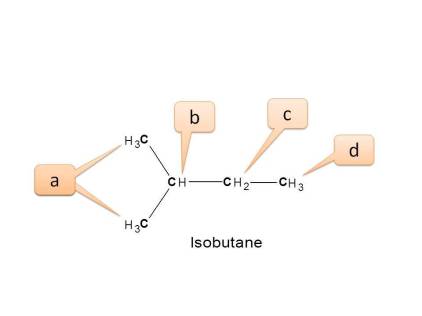
Step 1: Here three types of protons are present. Viz. CH3-, CH2-, CH-
- Here all methyl protons are not equivalent.
- Methyl protons indicated by “a” are attached to “-CH-”
- Methyl protons indicated by “d” are attached to “-CH 2-”
- Other non-equivalent nuclei are
- Methine protons (CH) ---- Indicated by “b”
- Methylene protons (CH 2) --- Indicated by “c”
Hence the total no. of NMR signals possible is “4”
Working Example 2: 2-bromo-4-chloro-3-methylpentane
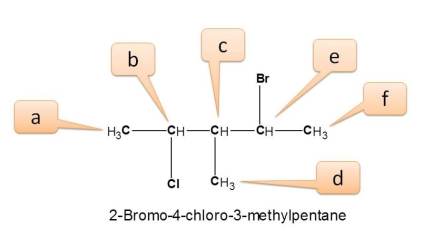
Here all protons are not equivalent and give different NMR signals.
- We can find three methyl protons indicated by a,d and f.
- a is attached –CHCl-
- d is attached by –CH-
- f is attached by –CHBr-
- Similarly three types of –CH protons indicated by b,c and e.
- b is attached to –Cl-
- c is attached to –CH3
- e is attached to –Br-
Hence total number of NMR signals is 6.
Working Example 3: Nitro benzene
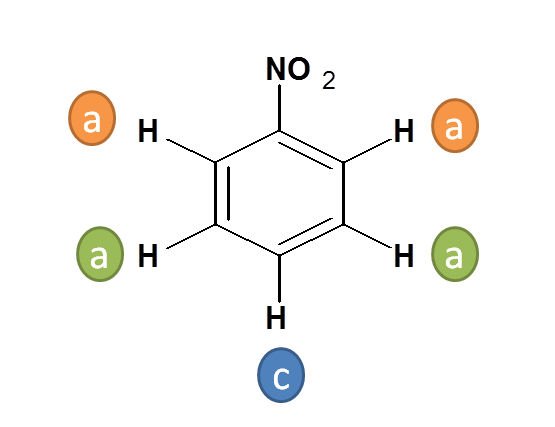
- Here three types of protons are present
- Two Protons indicated by “a” are ortho to nitro (-NO 2 ) group
- Two Protons indicated by “b” are meta to nitro (-NO 2 ) group
- One Proton indicated by “c” is para to nitro (-NO 2 ) group
- Hence the total no. of NMR signals possible is “3”
Here is a video explaining how to determine the number of NMR peaks along with examples.
In this way, we can determine the number of peaks possible for a compound in proton NMR by counting all the protons with different chemical or magnetic environment.