- Home >
- Test papers >
MCQ on UV-Visible spectroscopy: Page-12
(A) Compound A can be detected at very low concentrations than compound B.
(B) Compound B can be detected at very low concentrations than compound A.
(C) Both compounds can be detected at very dilute concentrations.
(D) Molar absorptivity have no influence on the detection of compounds.
(A) Water
(B) Ethanol
(C) Ethylene
(D) Chloroform
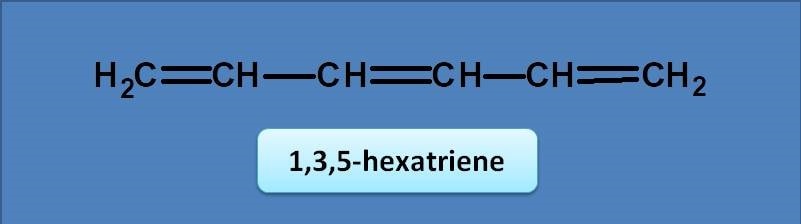
(A) 246 nm
(B) 245 nm
(C) 250 nm
(D) 247 nm
Normally diene has base value of 217 nm. Since the given compound is a triene, an extended conjugation produces an increment of 30 nm. Therefore the final value will be 217 + 30=247 nm.
(A) Same as that the substance emits in flame
(B) Same as that the substance absorbs
(C) Not same as that the substance absorbs
(D) Not same as that the substance emits
(A) n→π *
(B) π→π *
(C) d →d*
(D) n→s*