- Home >
- Test papers >
MCQ on UV-Visible spectroscopy: Page-8
(A) Fluorescence
(B) Phosphorescence
(C) IR
(D) NMR
Elections are paired in an orbital with opposite spins according to Pauli’s exclusion principle. This rule is applied to the electrons when they are in both ground and excited state. But sometimes the electron in the excited state may undergo a spin change such that it has same spin as the electron in the ground state. This electron now termed as in triplet state jumps to ground state by releasing energy in the form of phosphorescence.
(A) Fluorescence
(B) Analyte Dissociation
(C) Polychromatic radiation is used as source
(D) Analyte at high concentrations
Analyte when used at high concentration may result in non-linearity and deviation from Beer-Lambert’s law. This is mainly due to reduction of the speed of the light by the analyte compared to vacuum resulting in non-linearity.
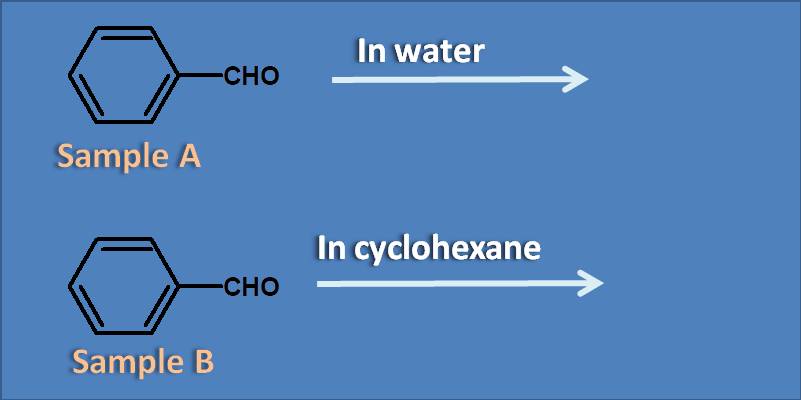
(A) Water increases pi to pi transition
(B) Cylcohexane increases pi to pi transition
(C) Both water and cyclohexane increase pi to pi transition
(D) Water increases n to pi transition while cyclohexane increases pi to pi transition
Since the analyte is same in both samples, the relative differences in the absorption of the samples only depends on the solvent. Generally a polar solvent like water can show positive solvatochromism on pi to pi transition therefore water will increase pi to pi transition. Similarly, non-polar solvents like cyclohexane show positive solvatochromism on n to pi transition.
(A) Sample A shows more absorption than sample B
(B) Sample B shows more absorption than sample A
(C) Sample A shows more absorption while sample B shows no absorption
(D) Sample A shows equal absorption as sample B
As benzaldehyde mainly shows stronger pi to pi transition, the solvent which increases this transition results in more absorption. Since water is a polar solvent that shows positive solvatochromism on pi to pi transition, sample A will more absorption than sample B.
(A) Absence of absorbance
(B) Hypsochromic shift
(C) Bathochromic shift
(D) No effect