- Home >
- Test papers >
MCQ on UV-Visible spectroscopy: Page-9
(A) lit.mol-1.cm-1
(B) dl.g -1cm -1
(C) No units
(D) lit.g -1cm -1
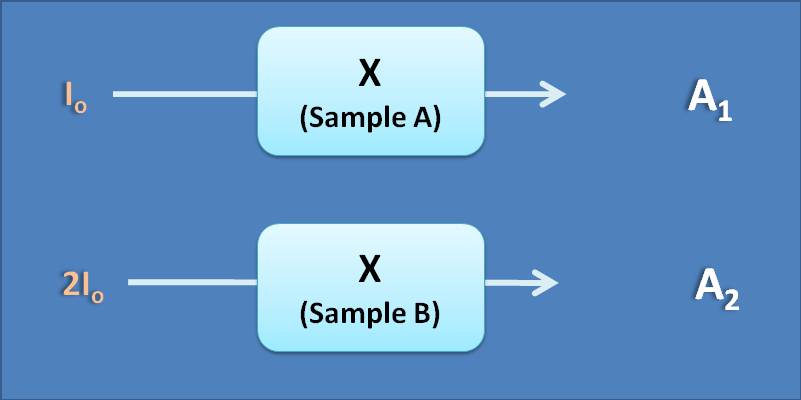
(A) A 2=4 A 1
(B) A 2=2 A 1
(C) A 2=A 1
(D) A 2=1/2 A 1
Absorbance is independent of intensity of the incident radiation as it is the ratio between intensities of incident radiation (I o) and transmitted radiations (I t). If you double I o, then I t will also be doubled proportionally making absorbance remains constant.
(A) Absorptivity changes with the intensity of the light
(B) Absorbance is independent of intensity of the light
(C) Absorbance has no units
(D) Absorptivity is a constant and depends on the nature of the molecule
Absorptivity is the intrinsic property of a molecule and it will be a constant at a specific temperature and wavelength of radiation. So it doesn’t change with intensity of incident radiation at a particular wavelength. that
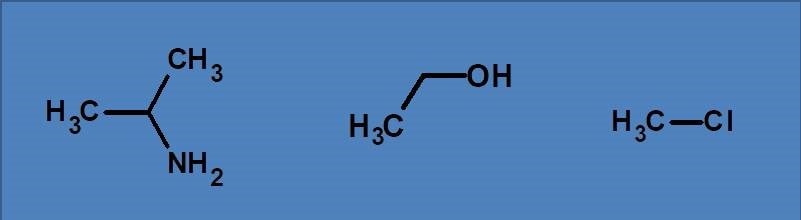
(A) n→π*, π→π*
(B) σ→σ*, n→π*, π→π*
(C) σ→σ*, n→σ*
(D) n→π*, n→σ*
All the listed compounds contain similar type of electrons such as sigma and n electrons. Therefore they can produce two types of transitions such as s→s* and n→s*. Both these transitions fall below 200 nm therefore show absorption only in vacuum UV region. These compounds can’t show p→p* and n→p* as they don’t have pi electrons.
(A) Vacuum UV region
(B) UV-visible region
(C) Visible only
(D) UV only
Since they doesn’t absorb in UV-visible region, they are transparent and colourless in this region. For a compound to absorb in UV-Visible region, it should have pi electrons.