- Home >
- Test papers >
MCQ on UV-Visible spectroscopy: Page-6
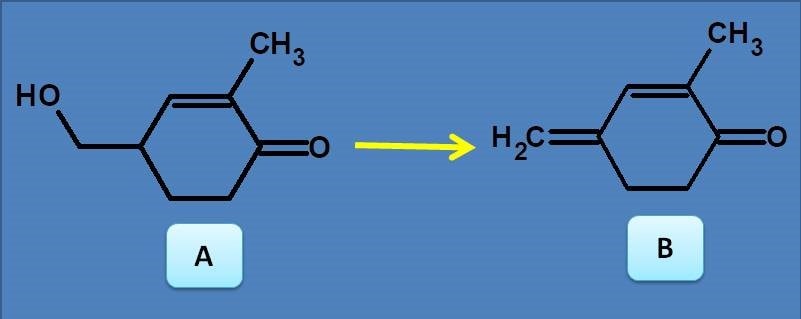
(A) Bathochromic shift
(B) Hypsochromic shift
(C) Bathochromic shift and hypochromism
(D) Hypsochromic shift and hyperchromism
We can easily observe that Compound B has extended conjugation compared with compound A. Therefore the spectra of B shows bathochromic shift and hyperchromism.
(A) 241
(B) 215
(C) 255
(D) 184
Wavelength range 200-400 nm indicates UV region and 400-800 nm indicates visible region. UV region can also be extended below 200 nm which is generally termed as vacuum UV but not suitable for practical purpose in UV spectrophotometers as many of the solvents also absorb and interfere with study. Therefore wavelength range from 200-800 nm is called as UV-visible region for practical purpose.
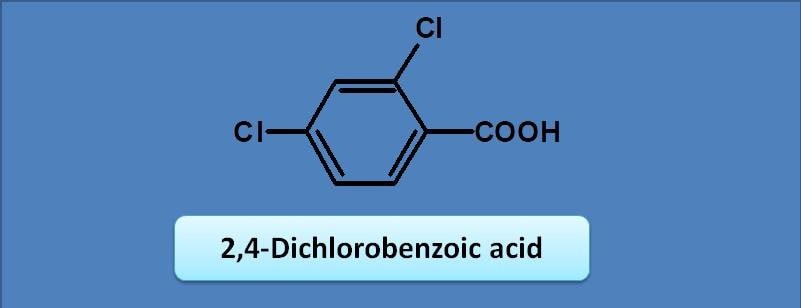
(A) 240 nm
(B) 250 nm
(C) 230 nm
(D) 217 nm
Here benzoic acid is the chromophore with base value as 230 nm. Two chlorine groups at ortho and para positions act as auxochromes with increments of 0 and 10 respectively. Therefore the final calculated value will be 230 + 10=240 nm.
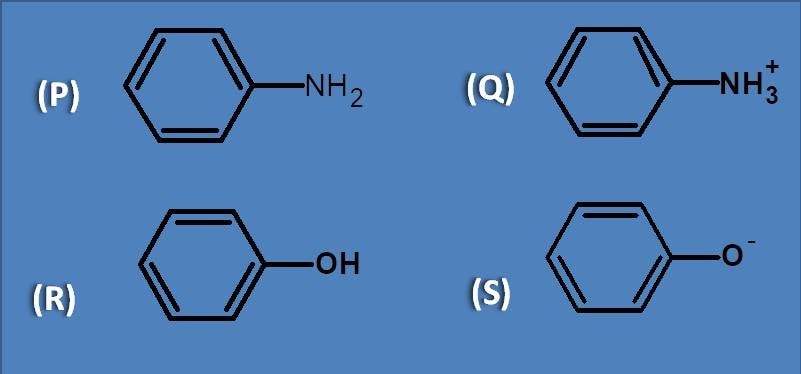
(A) Phenol
(B) Aniline
(C) Phenoxide ion
(D) Anilinium ion
All these are aromatic compounds and undergo pi to pi transition. Therefore the group that has n electrons and facilitates the pi to pi transition will have more bathochromic shift. Here the phenoxide ion has more number of lone pair of electrons, hence shows greater bathochromic shift.

(A) Q,R
(B) P,S
(C) P only
(D) P,Q,R and S
Bathochromic shift is produced by extended conjugation either by lone pair of electrons or by pi bond in the carbonyl group. So, any group whether electron withdrawing or electron donating can produce a bathochromic shift on benzene.