- Home >
- Test papers >
MCQ on UV-Visible spectroscopy: Page-5
(A) 5
(B) 10
(C) 11
(D) 18
Carotene is the important component in the carrot that has 11 conjugated double bonds producing a strong chromophore.
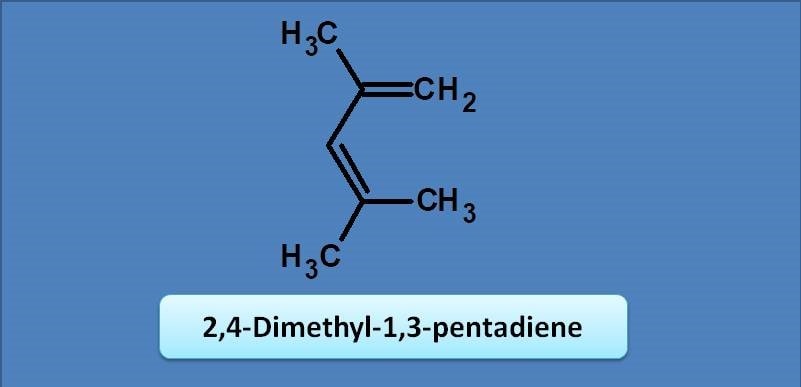
(A) 234
(B) 273
(C) 232
(D) 241
Since it is an acyclic diene, base value is 217 nm. One methyl group is at 2nd position and two methyl groups are at 4th position giving an increment of 3 x 5=15 nm. So the final value will be 217 + 15=232 nm.
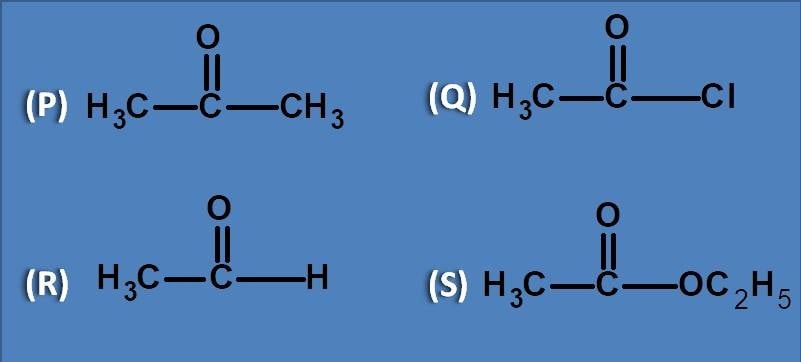
(A) Acetone
(B) Acetyl chloride
(C) Ethyl acetate
(D) Acetaldehyde
All the listed compounds have carbonyl group as chromophore with different groups attached to it. So, absorbance of the above compounds depends on the satiability of carbonyl group in its excited state. Since the carbon in pi to pi* transition gets partial positive charge, any group that destabilises will show poor absorption. Among the given compounds, acetaldehyde has least electronegative element as hydrogen therefore it will have least effect on stability and hence shows greater absorption.
(A) n→π*is forbidden
(B) π →π*is forbidden
(C) Both n→π*and π →π*are forbidden
(D) n→π*is forbidden but π →π*is allowed
Enones can undergo both pi to pi* and n to pi* transitions. Generally pi to pi transition in enones is so strong that practically n to pi transition is forbidden.
(A) UV-Visible spectroscopy
(B) IR spectroscopy
(C) Fluorescence spectroscopy
(D) X-ray diffraction
Both UV-visible and IR spectroscopy deal with absorption of the radiation by the analyte. On the other hand, Fluorescence spectroscopy involves measurement of radiation emitted by the analyte after excitation. So this technique is associated with molecular emission. Finally X-ray diffraction is a special technique where neither absorption nor emission is studied instead diffraction of incident radiation by the elements in the analyte is observed.