- Home >
- Test papers >
MCQ on UV-Visible spectroscopy: Page-1
(A) 400-800 nm
(B) 200-800 nm
(C) 25 μm-2.5 μm
(D) 2.5 μm – 1mm
Wavelength range 200-400 nm indicates UV region and 400-800 nm indicates visible region. UV region can also be extended below 200 nm which is generally termed as vacuum UV but not suitable for practical purpose in UV spectrophotometers as many of the solvents also absorb and interfere with study. Therefore wavelength range from 200-800 nm is called as UV-visible region for practical purpose.

(A) P,Q,R
(B) Q,R
(C) P,S
(D) P,Q,R,S
First of let’s see the possibilities for a compound with molecular formula C2H6O. Since it has oxygen it may be aldehyde, ketone, alcohol or ether. By matching with molecular formula, it can be concluded as acetaldehyde. 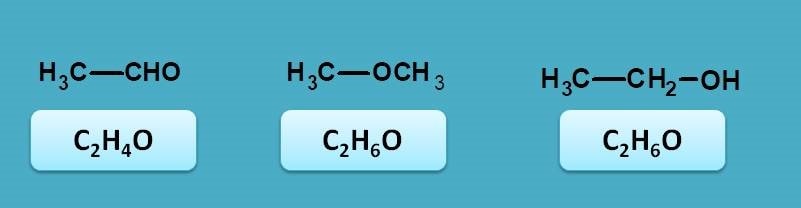
(A) n→p*
(B) s→s*
(C) p→p*
(D) n→s*, s→s*
Since the above compound is acetaldehyde, on reduction it gives ethanol. Now ethanol contains only sigma and n electrons therefore it shows sigma to sigma and n to sigma transitions. Both of these transitions fall within vacuum UV region.
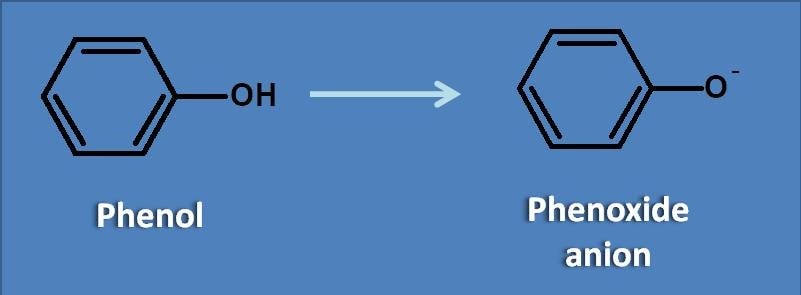
(A) Phenol shows more absorption than phenoxide anion
(B) Phenoxide anion shows more absorption than phenol
(C) Phenol has equal absorption as phenoxide anion
(D) Phenol shows absorption but phenoxide anion doesn’t
Generally absorption of a chromophore is increased by few of the groups like -OH, -NH2which are called as auxochromoes that facilitate absorption by the supplying lone pair of electrons in them. So more the number of lone pair of electrons, more will be the auxochromic effect. Here phenol has oxygen with two lone pair of electrons while phenoxide anion has oxygen with three lone pair of electrons. 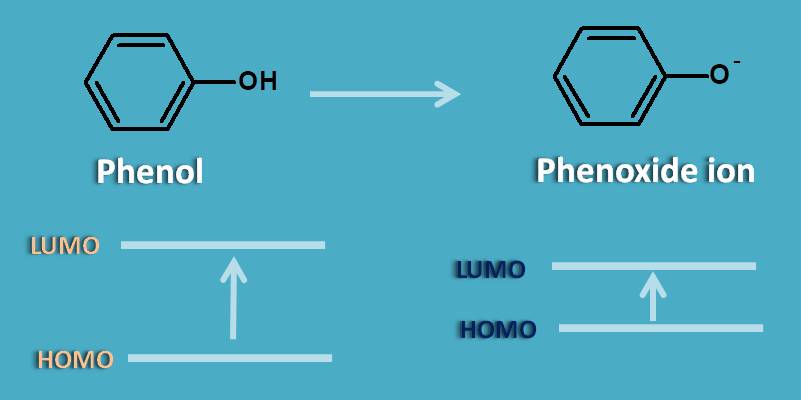

(A) Q,R
(B) P,R
(C) Q,S
(D) P,S
By increasing the pH to alkaline side, phenol exists as phenoxide ion which shows more absorption due to extra lone pair of electrons participating in pi to pi transition. This results in decrease energy gap between HOMO and LUMO of phenoxide ion. As the energy gap decreases, the wavelength of absorption increases as the two parameters are inversely proportional to each other.
Therefore it results in bathochromic shift or red shift.